Search Thermo Fisher Scientific
Thermo Scientific Chemicals
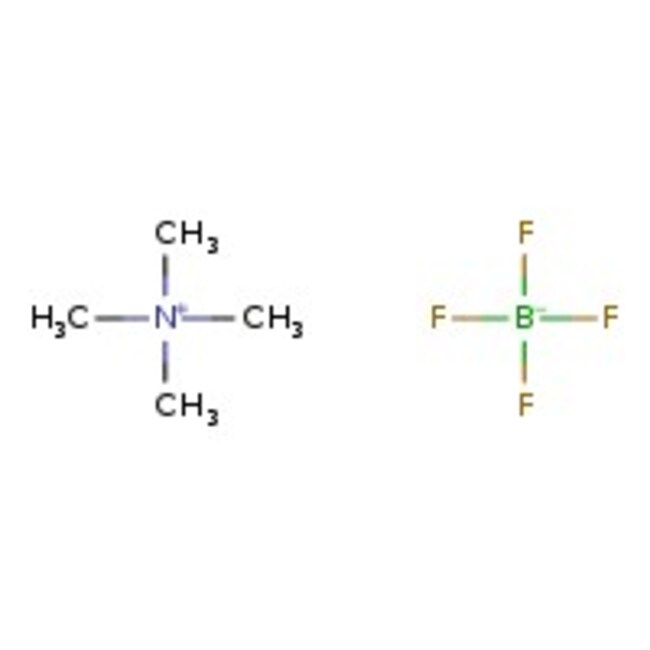
Tetramethylammonium tetrafluoroborate, 97%, Thermo Scientific Chemicals
Catalog number A17436.22
also known as A17436-22
Price (USD)/ Each
192.65
Online exclusive
214.00 Save 21.35 (10%)
-
Quantity:
100 g
Price (USD)/ Each
192.65
Online exclusive
214.00 Save 21.35 (10%)
Tetramethylammonium tetrafluoroborate, 97%, Thermo Scientific Chemicals
Catalog numberA17436.22
Price (USD)/ Each
192.65
Online exclusive
214.00 Save 21.35 (10%)
-
Chemical Identifiers
CAS661-36-9
IUPAC Nametetrafluoroboranuide; tetramethylazanium
Molecular FormulaC4H12BF4N
InChI KeyXWFABLFRYCHILB-UHFFFAOYSA-N
SMILESC[N+](C)(C)C.F[B-](F)(F)F
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White
Assay from Supplier's CofA≥96.0 to ≤104.0% (based on elemental analysis)
FormCrystals or powder or crystalline powder and/or chunks
It is used in the catalytic reduction of 1,6-dihalohexanes by nickel(i) salen electrogenerated at glassy carbon cathodes in dimethylformamide.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is used in the catalytic reduction of 1,6-dihalohexanes by nickel(i) salen electrogenerated at glassy carbon cathodes in dimethylformamide.
Solubility
Solubility in hot water is almost transparent.
Notes
Hygroscopic. Store away from water/moisture and oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition. Store under inert gas. Protect from humidity.
It is used in the catalytic reduction of 1,6-dihalohexanes by nickel(i) salen electrogenerated at glassy carbon cathodes in dimethylformamide.
Solubility
Solubility in hot water is almost transparent.
Notes
Hygroscopic. Store away from water/moisture and oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition. Store under inert gas. Protect from humidity.
RUO – Research Use Only
General References:
- Christopher E. Dahm, et al. Electrochemical and spectroscopic characterization of anodically formed nickel salen polymer films on glassy carbon, platinum, and optically transparent tin oxide electrodes in acetonitrile containing tetramethylammonium tetrafluoroborate.J. Electroanal. Chem.,1996,410(2), 163-171.
- Parichatr Vanalabhpatana, et al. Catalytic Reduction of 1,6-Dihalohexanes by Nickel(I) Salen Electrogenerated at Glassy Carbon Cathodes in Dimethylformamide.J. Electrochem. Soc.,2005,152(7), 222-E229.