Search Thermo Fisher Scientific
Thermo Scientific Chemicals
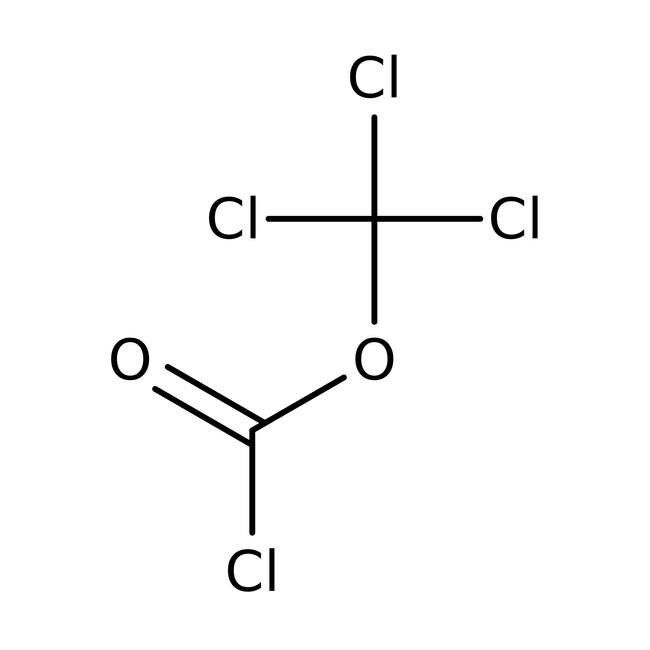
Trichloromethyl chloroformate, 98%, Thermo Scientific Chemicals
Catalog number A17444.18
also known as A17444-18
Price (USD)/ Each
206.65
Online exclusive
229.00 Save 22.35 (10%)
-
Quantity:
50 g
Price (USD)/ Each
206.65
Online exclusive
229.00 Save 22.35 (10%)
Trichloromethyl chloroformate, 98%, Thermo Scientific Chemicals
Catalog numberA17444.18
Price (USD)/ Each
206.65
Online exclusive
229.00 Save 22.35 (10%)
-
Chemical Identifiers
CAS503-38-8
IUPAC Nametrichloromethyl carbonochloridate
Molecular FormulaC2Cl4O2
InChI KeyHCUYBXPSSCRKRF-UHFFFAOYSA-N
SMILESClC(=O)OC(Cl)(Cl)Cl
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless
Assay (Titration ex Chloride)≥97.5 to ≤102.5%
Refractive Index1.4560-1.4600 @ 20?C
Identification (FTIR)Conforms
FormLiquid
Trichloromethyl chloroformate is used as a reagent in the synthesis of organic compounds. It serves as a source of phosgene used in some laboratory preparations. Also, it is used as a reactant for the synthesis of cyclic carbamimidates, N-alkenyl and cycloalkyl carbamates and prostate-specific membrane antigen-targeted anticancer prodrugs. In addition, it is involved in the preparation of an erythromycin A derivatives and antibody-drug conjugates. It is utilized in the conversion of amines, carboxylic acids, formamides in to isocyanates, acid chlorides and isocyanides respectively.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Trichloromethyl chloroformate is used as a reagent in the synthesis of organic compounds. It serves as a source of phosgene used in some laboratory preparations. Also, it is used as a reactant for the synthesis of cyclic carbamimidates, N-alkenyl and cycloalkyl carbamates and prostate-specific membrane antigen-targeted anticancer prodrugs. In addition, it is involved in the preparation of an erythromycin A derivatives and antibody-drug conjugates. It is utilized in the conversion of amines, carboxylic acids, formamides in to isocyanates, acid chlorides and isocyanides respectively.
Solubility
Miscible with ethanol, ether and benzene. Immiscible with water.
Notes
Special handling precautions required. View MSDS prior to purchase. MSDS are available online at www.alfa.comStore in a cool place. Light and heat sensitive. Incompatible with strong oxidizing agents and bases.
Trichloromethyl chloroformate is used as a reagent in the synthesis of organic compounds. It serves as a source of phosgene used in some laboratory preparations. Also, it is used as a reactant for the synthesis of cyclic carbamimidates, N-alkenyl and cycloalkyl carbamates and prostate-specific membrane antigen-targeted anticancer prodrugs. In addition, it is involved in the preparation of an erythromycin A derivatives and antibody-drug conjugates. It is utilized in the conversion of amines, carboxylic acids, formamides in to isocyanates, acid chlorides and isocyanides respectively.
Solubility
Miscible with ethanol, ether and benzene. Immiscible with water.
Notes
Special handling precautions required. View MSDS prior to purchase. MSDS are available online at www.alfa.comStore in a cool place. Light and heat sensitive. Incompatible with strong oxidizing agents and bases.
RUO – Research Use Only
General References:
- Convenient replacement for phosgene in many reactions, for example:
- Conversion of amines to isocyanates: J. Org. Chem., 41, 2070 (1976); Org. Synth. Coll ., 6, 715 (1988). For aliphatic amines, best results were obtained in the presence of the hindered amine 1,8-Bis(dimethyl amino) naphthalene, L00313: J. Org. Chem., 61, 3883 (1996).
- Dehydration of N-monosubstituted formamides to isocyanides: Angew. Chem. Int. Ed., 16, 259 (1977).
- Formation of nitriles from oximes Synthesis, 1037 (1986); or carboxamides: Tetrahedron Lett., 27, 2203 (1986).
- See also Triphosgene, A14932, and Oxalyl chloride, A18012.
- Lezama, J.; Domínguez, R. M.; Chuchani, G. Kinetics of the Gas-Phase Elimination Reaction of Benzyl Chloroformate and Neopentyl Chloroformate. Int. J. Chem. Kinet. 2015, 47 (2), 104-112.
- Prousis, K. C.; Markopoulos, J.; Mckee, V.; Igglessi-Markopoulou, O. An efficient synthetic approach towards fully functionalized tetronic acids: the use of 1, 3-dioxolane-2, 4-diones as novel protected-activated synthons of α-hydroxy acids. Tetrahedron 2015, 71 (45), 8637-8648.