Search Thermo Fisher Scientific
Thermo Scientific Chemicals
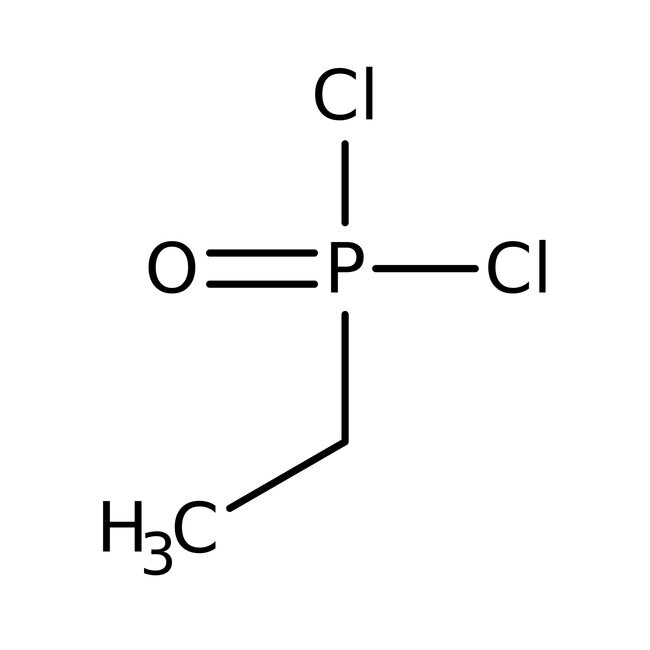
Ethylphosphonic dichloride, 98%, Thermo Scientific Chemicals
Chemical Identifiers
CAS1066-50-8
IUPAC Nameethylphosphonoyl dichloride
Molecular FormulaC2H5Cl2OP
InChI KeyOWGJXSYVHQEVHS-UHFFFAOYSA-N
SMILESCCP(Cl)(Cl)=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless
Refractive Index1.4645-1.4685 @ 20?C
FormLiquid
Assay (GC)≥97.5%
Ethylphosphonic dichloride finds it uses in the preparation of: ethylphosphonic diisocyanate, 4-acetylphenyl isopropyl ethylphosphonate, 4-acetylphenyl phenyl ethylphosphonate and 4-acetylphenyl cyclohexyl ethylphosphonate.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Ethylphosphonic dichloride finds it uses in the preparation of: ethylphosphonic diisocyanate, 4-acetylphenyl isopropyl ethylphosphonate, 4-acetylphenyl phenyl ethylphosphonate and 4-acetylphenyl cyclohexyl ethylphosphonate.
Solubility
Soluble in chloroform and dichloromethane.
Notes
Moisture Sensitive. Store away from oxidizing agents, water/moisture. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
Ethylphosphonic dichloride finds it uses in the preparation of: ethylphosphonic diisocyanate, 4-acetylphenyl isopropyl ethylphosphonate, 4-acetylphenyl phenyl ethylphosphonate and 4-acetylphenyl cyclohexyl ethylphosphonate.
Solubility
Soluble in chloroform and dichloromethane.
Notes
Moisture Sensitive. Store away from oxidizing agents, water/moisture. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
RUO – Research Use Only
General References:
- Charity Nowlan.; Yingchun Li.; Johannes C Hermann.; Timothy Evans.; Joseph Carpenter.; Eman Ghanem.; Brian K Shoichet.; Frank M Raushel. Resolution of chiral phosphate, phosphonate, and phosphinate esters by an enantioselective enzyme library.J Am Chem Soc.,2006,128(49), 15892-15902.
- A. C. HavenJr. Organophosphorus Isocyanates.J. Am. Chem. Soc.,1956,78(4), 842-843.
- R. Quinn.; J. B. Appleby & G. P. Pez. Hydrogen sulfide separation from gas streams using salt hydrate chemical absorbents and immobilized liquid membranes.Sep. Sci. Technol.;2002,37(3), 627-638.