Search Thermo Fisher Scientific
Thermo Scientific Chemicals
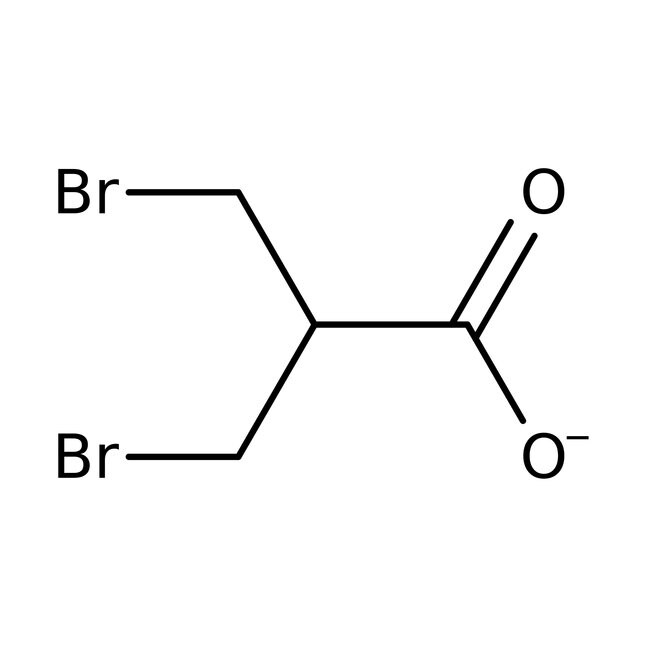
3-Bromo-2-(bromomethyl)propionic acid, 98%, Thermo Scientific Chemicals
Catalog number A19630.14
also known as A19630-14
Price (USD)/ Each
233.65
Online exclusive
259.00 Save 25.35 (10%)
-
Quantity:
25 g
Price (USD)/ Each
233.65
Online exclusive
259.00 Save 25.35 (10%)
3-Bromo-2-(bromomethyl)propionic acid, 98%, Thermo Scientific Chemicals
Catalog numberA19630.14
Price (USD)/ Each
233.65
Online exclusive
259.00 Save 25.35 (10%)
-
Chemical Identifiers
CAS41459-42-1
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to cream to brown
FormCrystals or powder or crystalline powder
Assay (Aqueous acid-base Titration)≥97.5 to ≤102.5%
Melting Point (clear melt)96.0-102.0?C
3-Bromo-2-(bromomethyl)propionic acid acts as an organic building block for the preparation of beta-substituted acrylates. It is used in the preparation of t-butyl 2-(phenylthiomethyl) propenoate, t-butyl 3-(phenylthio)-2-(phenylthiomethyl)propenoate and 3-(phenylthio)-2-(phenyl-sulfinylmethyl)propenoate. Further, it plays an important role in the synthesis of beta-lactams by cyclization of the corresponding amide.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
3-Bromo-2-(bromomethyl)propionic acid acts as an organic building block for the preparation of beta-substituted acrylates. It is used in the preparation of t-butyl 2-(phenylthiomethyl) propenoate, t-butyl 3-(phenylthio)-2-(phenylthiomethyl)propenoate and 3-(phenylthio)-2-(phenyl-sulfinylmethyl)propenoate. Further, it plays an important role in the synthesis of beta-lactams by cyclization of the corresponding amide.
Solubility
Soluble in acetic acid.
Notes
Incompatible with strong oxidizing agents and strong bases.
3-Bromo-2-(bromomethyl)propionic acid acts as an organic building block for the preparation of beta-substituted acrylates. It is used in the preparation of t-butyl 2-(phenylthiomethyl) propenoate, t-butyl 3-(phenylthio)-2-(phenylthiomethyl)propenoate and 3-(phenylthio)-2-(phenyl-sulfinylmethyl)propenoate. Further, it plays an important role in the synthesis of beta-lactams by cyclization of the corresponding amide.
Solubility
Soluble in acetic acid.
Notes
Incompatible with strong oxidizing agents and strong bases.
RUO – Research Use Only
General References:
- Zhang, X.; Teixeira, V.; Porcal, W.; Cabral, P.; Gambini, J. P.; Fernandez, M.; Gallazzi, F.; Quinn, T. P. [99mTc(CO)3]+ and [99mTcO2]+ Radiolabeled Cyclic Melanotropin Peptides for Melanoma SPECT Imaging. Curr. Radiopharm. 2014, 7 (1), 63-74.
- Hicks, M. R.; Rullay, A. K.; Pedrido, R.; Crout, D. H.; Pinheiro, T. J. T. Efficient Synthesis of Methanesulphonate-Derived Lipid Chains for Attachment of Proteins to Lipid Membranes. Synth. Commun. 2008, 38 (21), 3726-3750.