Search Thermo Fisher Scientific
Thermo Scientific Chemicals
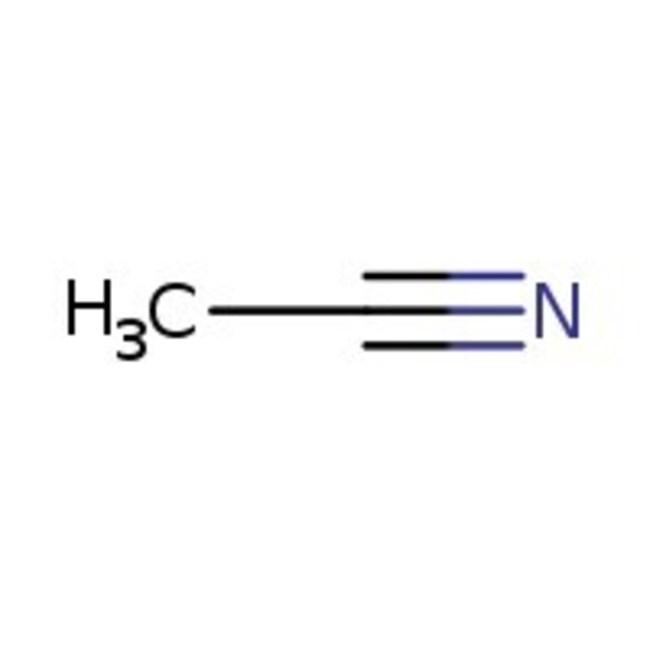
Acetonitrile, 99%, Thermo Scientific Chemicals
Catalog number A19862.0F
also known as A19862-0F
Price (USD)/ Each
122.65
Online exclusive
136.00 Save 13.35 (10%)
-
Quantity:
2500 mL
Price (USD)/ Each
122.65
Online exclusive
136.00 Save 13.35 (10%)
Acetonitrile, 99%, Thermo Scientific Chemicals
Catalog numberA19862.0F
Price (USD)/ Each
122.65
Online exclusive
136.00 Save 13.35 (10%)
-
Chemical Identifiers
CAS75-05-8
IUPAC Nameacetonitrile
Molecular FormulaC2H3N
InChI KeyWEVYAHXRMPXWCK-UHFFFAOYSA-N
SMILESCC#N
View more
Specifications Specification Sheet
Specification Sheet
Assay (GC)≥98.5%
Appearance (Color)Clear colorless
Refractive Index1.3425-1.3455 @ 20?C
FormLiquid
Identification (FTIR)Conforms
Acetonitrile is utilized as a polar aprotic solvent in organic synthesis, and in the purification of butadiene. It is used in all the analytical laboratories as a major component of mobile phase in High Performance Liquid Chromatography (HPLC) and in Liquid Chromatography - Mass Spectrometry (LC-MS). It is used for the extraction of fatty acids, spinning fibers and casting and molding of plastic materials. Aqueous two-phase systems based on acetonitrile and carbohydrates play an important character in the extraction and purification of biomolecules called vanillins. It acts as a stabilizer for the chlorinated solvents, and finds use in the production of DNA oligonucleotides.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Acetonitrile is utilized as a polar aprotic solvent in organic synthesis, and in the purification of butadiene. It is used in all the analytical laboratories as a major component of mobile phase in High Performance Liquid Chromatography (HPLC) and in Liquid Chromatography - Mass Spectrometry (LC-MS). It is used for the extraction of fatty acids, spinning fibers and casting and molding of plastic materials. Aqueous two-phase systems based on acetonitrile and carbohydrates play an important character in the extraction and purification of biomolecules called vanillins. It acts as a stabilizer for the chlorinated solvents, and finds use in the production of DNA oligonucleotides.
Solubility
Miscible with water, alcohols, ethers, acetone, chloroform, carbon tetrachloride and ethylene chloride. Immiscible with saturated hydrocarbons (petroleum fractions).
Notes
Incompatible with alkali metals, acids, bases, reducing agents and oxidizing agents. Highly flammable. Store it away from heat, sparks, and flame exposure.
Acetonitrile is utilized as a polar aprotic solvent in organic synthesis, and in the purification of butadiene. It is used in all the analytical laboratories as a major component of mobile phase in High Performance Liquid Chromatography (HPLC) and in Liquid Chromatography - Mass Spectrometry (LC-MS). It is used for the extraction of fatty acids, spinning fibers and casting and molding of plastic materials. Aqueous two-phase systems based on acetonitrile and carbohydrates play an important character in the extraction and purification of biomolecules called vanillins. It acts as a stabilizer for the chlorinated solvents, and finds use in the production of DNA oligonucleotides.
Solubility
Miscible with water, alcohols, ethers, acetone, chloroform, carbon tetrachloride and ethylene chloride. Immiscible with saturated hydrocarbons (petroleum fractions).
Notes
Incompatible with alkali metals, acids, bases, reducing agents and oxidizing agents. Highly flammable. Store it away from heat, sparks, and flame exposure.
RUO – Research Use Only
General References:
- Polar aprotic solvent for a wide variety of reactions, including nucleophilic substitutions, oxidations, reductions and organometallic reactions. Widely used with crown ethers for the generation of 'naked' anions from their salts; see, e.g. 18-Crown-6, A11249 . Preferred solvent for RuO4 oxidations, due to its coordinating ability; see Ruthenium(III) chloride hydrate, 11043 .
- Undergoes the Ritter reaction with alcohols or olefins in the presence of acid to give N-substituted acetamides. For benzylic alcohols, modified conditions employing boron trifluoride etherate give high yields: Synth. Commun., 24, 601 (1994). For an example, see 4-Methyl benzyl alcohol, A15315 .
- For use in the mild conversion of amides to nitriles, see Benzamide, A10501 .
- Cardoso, G. B.; Mourao, T.; Pereira, F. M.; Freire, M. G.; Fricks, A. T.; Soares, C. M. F.; Lima, A. S. Aqueous two-phase systems based on acetonitrile and carbohydrates and their application to the extraction of vanillin. Sep. Purif. Technol. 2013, 104 (5), 106-113.
- Xu, K.; Zhu, L.; Zhang, A.; Jiang, G.; Tang, H. A peculiar cyclic voltammetric behavior of polyaniline in acetonitrile and its application in ammonia vapor sensor. J. Electroanal. Chem. 2007, 608 (2), 141-147.
- Lam, T. W.; Zhang, H.; Siu, S. K. Reductions of Oxygen, Carbon Dioxide, and Acetonitrile by the Magnesium(II)/Magnesium(I) Couple in Aqueous Media: Theoretical Insights from a Nano-Sized Water Droplet. J. Phys. Chem. A 2015, 119 (12), 2780-2792.