Search Thermo Fisher Scientific
Thermo Scientific Chemicals
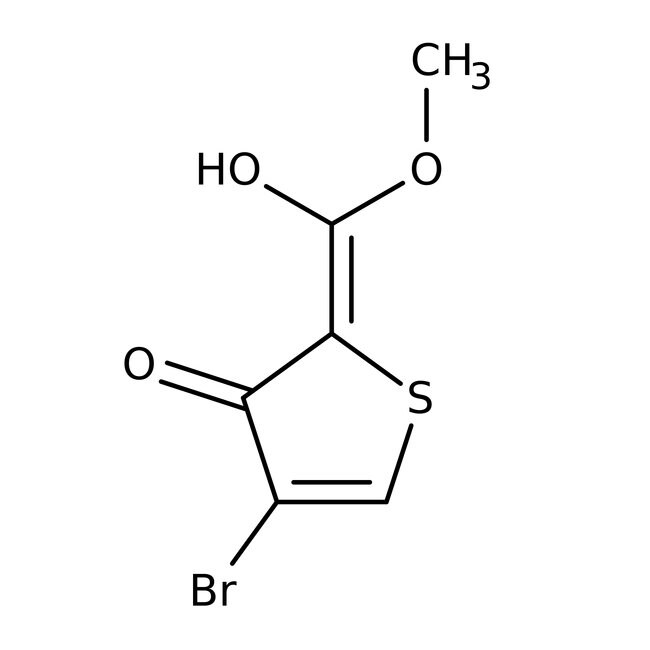
Methyl 4-bromo-3-hydroxythiophene-2-carboxylate, 97%, Thermo Scientific Chemicals
Catalog number B20249.09
also known as B20249-09
Price (USD)/ Each
277.00
-
Quantity:
10 g
Price (USD)/ Each
277.00
Methyl 4-bromo-3-hydroxythiophene-2-carboxylate, 97%, Thermo Scientific Chemicals
Catalog numberB20249.09
Price (USD)/ Each
277.00
-
Chemical Identifiers
CAS95201-93-7
IUPAC Name(2Z)-4-bromo-2-[hydroxy(methoxy)methylidene]-2,3-dihydrothiophen-3-one
Molecular FormulaC6H5BrO3S
InChI KeyYCTROWLDGFWISA-WAYWQWQTSA-N
SMILESCOC(O)=C1/SC=C(Br)C1=O
View more
Specifications Specification Sheet
Specification Sheet
Assay (GC)≥96.0%
Appearance (Color)White to cream or pale orange to pale brown or pink/brown to pink/red
FormPowder
Melting Point (clear melt)76-82?C
Identification (FTIR)Conforms
The synthesis of the thienopyranone scaffold began with the alkylation of methyl 4-bromo-3-hydroxythiophene-2-carboxylate with N-acetylmorpholine.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
The synthesis of the thienopyranone scaffold began with the alkylation of methyl 4-bromo-3-hydroxythiophene-2-carboxylate with N-acetylmorpholine.
Solubility
Sparingly Soluble in water 0.95 g/L @ 25°C.
Notes
Store in cool, dry place in a well sealed container. Store away from oxidizing agents, bases, reducing agents.
The synthesis of the thienopyranone scaffold began with the alkylation of methyl 4-bromo-3-hydroxythiophene-2-carboxylate with N-acetylmorpholine.
Solubility
Sparingly Soluble in water 0.95 g/L @ 25°C.
Notes
Store in cool, dry place in a well sealed container. Store away from oxidizing agents, bases, reducing agents.
RUO – Research Use Only
Guillermo A. Morales; Joseph R. Garlich; Jingdong Su; Xiaodong Peng; Jessica Newblom; Kevin Weber and Donald L. Durden. Synthesis and Cancer Stem Cell-Based Activity of Substituted 5-Morpholino-7H-thieno[3,2-b]pyran-7-ones Designed as Next Generation PI3K Inhibitors. J. Med. Chem. 2013, 56 (5), 1922-1939.