Search Thermo Fisher Scientific
Thermo Scientific Chemicals
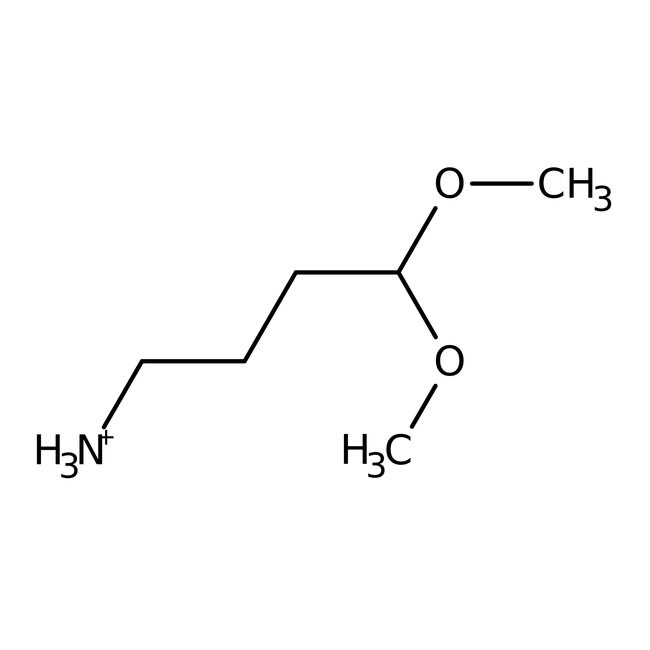
4-Aminobutyraldehyde dimethyl acetal, 98+%, Thermo Scientific Chemicals
Catalog number B21072.14
also known as B21072-14
Price (USD)/ Each
176.00
-
Quantity:
25 g
Price (USD)/ Each
176.00
4-Aminobutyraldehyde dimethyl acetal, 98+%, Thermo Scientific Chemicals
Catalog numberB21072.14
Price (USD)/ Each
176.00
-
Chemical Identifiers
CAS19060-15-2
IUPAC Name4,4-dimethoxybutan-1-aminium
Molecular FormulaC6H16NO2
InChI KeyTYVAXMOICMBSMT-UHFFFAOYSA-O
SMILESCOC(CCC[NH3+])OC
View more
Specifications Specification Sheet
Specification Sheet
FormLiquid
Assay (GC)≥98.0%
Appearance (Color)Clear colorless
Refractive Index1.4280-1.4320 @ 20?C
4-Aminobutyraldehyde dimethyl acetal acts as an intermediate in organic synthesis. It is used in the preparation of pineal hormone melatonin by reaction with 4-methoxyphenylhydrazine hydrochloride and acetic anhydride. Further, it is also used in the preparation of ficuseptine, juliprosine, and juliprosopine.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Aminobutyraldehyde dimethyl acetal acts as an intermediate in organic synthesis. It is used in the preparation of pineal hormone melatonin by reaction with 4-methoxyphenylhydrazine hydrochloride and acetic anhydride. Further, it is also used in the preparation of ficuseptine, juliprosine, and juliprosopine.
Solubility
Miscible with benzene.
Notes
Air sensitive. Incompatible with acids, acid chlorides, acid anhydrides, strong oxidizing agents and carbon dioxide.
4-Aminobutyraldehyde dimethyl acetal acts as an intermediate in organic synthesis. It is used in the preparation of pineal hormone melatonin by reaction with 4-methoxyphenylhydrazine hydrochloride and acetic anhydride. Further, it is also used in the preparation of ficuseptine, juliprosine, and juliprosopine.
Solubility
Miscible with benzene.
Notes
Air sensitive. Incompatible with acids, acid chlorides, acid anhydrides, strong oxidizing agents and carbon dioxide.
RUO – Research Use Only
General References:
- Dmitriev, M. E.; Ragulin, V. V. Synthesis of phosphorus isosters of beta-amyloid peptides fragments. Russ. J. Gen. Chem. 2015, 85 (9), 2091-2098.
- Bari, M. R.; Hassan, M.; Akai, N.; Arima, J.; Mori, N. Gene cloning and biochemical characterization of 4-N-trimethylaminobutyraldehyde dehydrogenase II from Pseudomonas sp. 13CM. World J. Microbiol. Biotechnol. 2013, 29 (4), 683-692.