Search Thermo Fisher Scientific
Thermo Scientific Chemicals
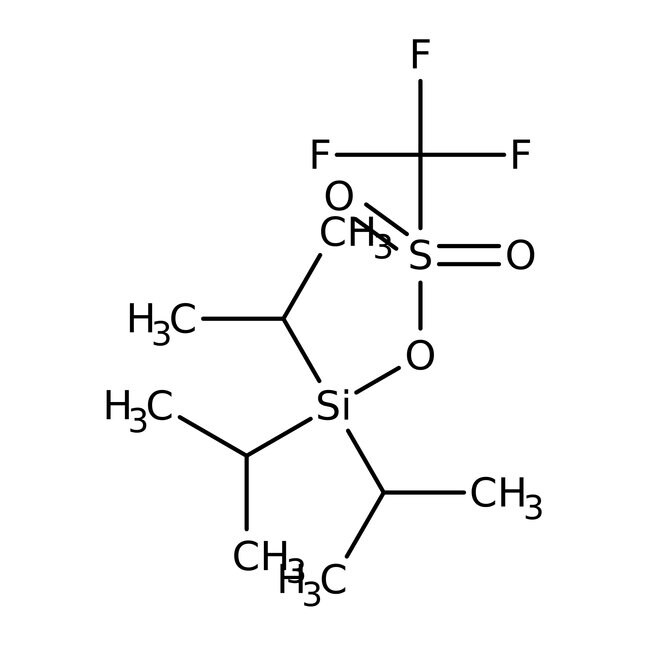
Triisopropylsilyl trifluoromethanesulfonate, 97%, Thermo Scientific Chemicals
Catalog number B21127.06
also known as B21127-06
Price (USD)/ Each
48.10
-
Quantity:
5 g
Price (USD)/ Each
48.10
Triisopropylsilyl trifluoromethanesulfonate, 97%, Thermo Scientific Chemicals
Catalog numberB21127.06
Price (USD)/ Each
48.10
-
Chemical Identifiers
CAS80522-42-5
IUPAC Nametris(propan-2-yl)silyl trifluoromethanesulfonate
Molecular FormulaC10H21F3O3SSi
InChI KeyLHJCZOXMCGQVDQ-UHFFFAOYSA-N
SMILESCC(C)[Si](OS(=O)(=O)C(F)(F)F)(C(C)C)C(C)C
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to yellow
FormLiquid
Assay (Aqueous acid-base Titration)≥96.0 to ≤104.0%
Identification (FTIR)Conforms
Refractive Index1.4120-1.4170 @ 20°C
Triisopropylsilyl trifluoromethanesulfonate is used as a reagent for the introduction of triisopropylsilyl(TIPS) group in organic synthesis. It is involved in the synthesis of 2-substituted benzothiopyran-4-ones and silyloxy acetylenes. As a protecting group in organic synthesis, it is utilized especially for the protection of primary amines. Furthermore, it plays an important role in Takahashi Taxol total synthesis or for chemical glycosylation reactions.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Triisopropylsilyl trifluoromethanesulfonate is used as a reagent for the introduction of triisopropylsilyl(TIPS) group in organic synthesis. It is involved in the synthesis of 2-substituted benzothiopyran-4-ones and silyloxy acetylenes. As a protecting group in organic synthesis, it is utilized especially for the protection of primary amines. Furthermore, it plays an important role in Takahashi Taxol total synthesis or for chemical glycosylation reactions.
Solubility
Miscible with chloroform and ethyl acetate.
Notes
Moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong bases and strong oxidizing agents. Hydrolyzes in water.
Triisopropylsilyl trifluoromethanesulfonate is used as a reagent for the introduction of triisopropylsilyl(TIPS) group in organic synthesis. It is involved in the synthesis of 2-substituted benzothiopyran-4-ones and silyloxy acetylenes. As a protecting group in organic synthesis, it is utilized especially for the protection of primary amines. Furthermore, it plays an important role in Takahashi Taxol total synthesis or for chemical glycosylation reactions.
Solubility
Miscible with chloroform and ethyl acetate.
Notes
Moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong bases and strong oxidizing agents. Hydrolyzes in water.
RUO – Research Use Only
General References:
- Reagent for introduction of the triisopropylsilyl (TIPS) group: Tetrahedron Lett., 22, 3455 (1981); compare Chlorotriisopropyl silane, A17376, and Appendix 4. For general reactions of silyl triflates, see Trimethyl silyl trifluoromethanesulfonate, A12535.
- For use in the synthesis of 2-substituted benzothiopyran-4-ones, see: Synlett, 182 (1996):
- A method for protecting both H atoms of a primary amine consists of formation of the succinimide, followed by enolsilylation with TIPS-OTf, to form the 2,5-bis(triisopropylsiloxy)pyrroles. Deprotection can be effected by desilylation with dilute HCl, followed by hydrazinolysis: Tetrahedron Lett., 38, 2617 (1997).
- For a comprehensive review of the TIPS group in organic chemistry, see: Chem. Rev., 95, 1009 (1995).
- Hayashi, Y.; Yamazaki, T.; Nakanishi, Y.; Ono, T.; Taniguchi, T.; Monde, K.; Uchimaru, T. Asymmetric Nitrocyclopropanation of alfa-Substituted alfa, beta-Enals Catalyzed by Diphenylprolinol Silyl Ether for the Construction of All-Carbon Quaternary Stereogenic Centers. Eur. J. Org. Chem. 2015, 2015 (26), 5747-5754.
- Chang, J. C.; Lai, C. C.; Chiu, S. H. A Redox-Controllable Molecular Switch Based on Weak Recognition of BPX26C6 at a Diphenylurea Station. Molecules 2015, 20 (2), 1775-1787.