Search Thermo Fisher Scientific
Thermo Scientific Chemicals
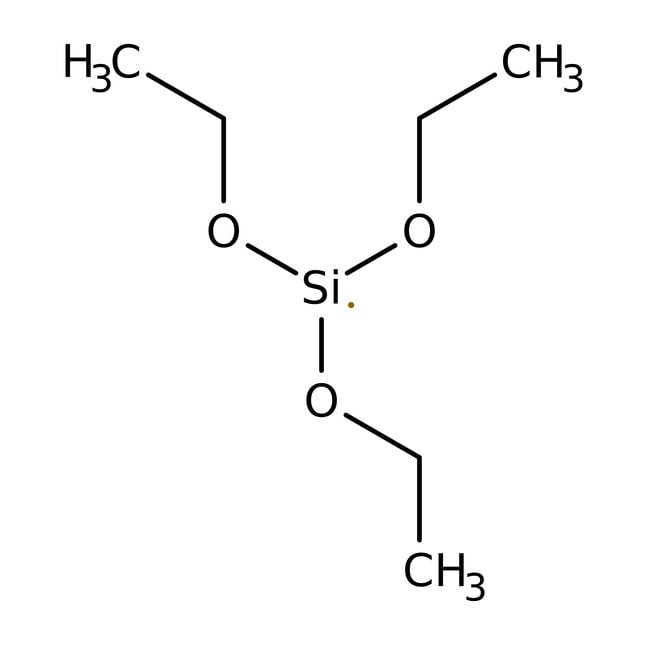
Triethoxysilane, 96%, Thermo Scientific Chemicals
Catalog number B22063.30
also known as B22063-30
Price (USD)/ Each
441.65
Online exclusive
490.00 Save 48.35 (10%)
-
Quantity:
250 g
Price (USD)/ Each
441.65
Online exclusive
490.00 Save 48.35 (10%)
Triethoxysilane, 96%, Thermo Scientific Chemicals
Catalog numberB22063.30
Price (USD)/ Each
441.65
Online exclusive
490.00 Save 48.35 (10%)
-
Chemical Identifiers
CAS998-30-1
IUPAC Nametriethoxysilyl
Molecular FormulaC6H15O3Si
InChI KeyPKDCQJMRWCHQOH-UHFFFAOYSA-N
SMILESCCO[Si](OCC)OCC
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless
FormLiquid
Assay (GC)≥95.0% (non-U.S. specification)
Assay from Suppliers CofA≥95.0% (GC, U.S. specification)
CommentSpecification differs for U.S. and non-U.S. material where indicated
View more
Triethoxysilane is raw material for synthesis of silane addition agent and silicon oil. It is used as a reducing agent.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Triethoxysilane is raw material for synthesis of silane addition agent and silicon oil. It is used as a reducing agent.
Solubility
Insoluble in water. Soluble in organic solvents.
Notes
Moisture sensitive. Store away from strong oxidizing agents, strong acids, bases, water/ moisture. Store under inert gas.
Triethoxysilane is raw material for synthesis of silane addition agent and silicon oil. It is used as a reducing agent.
Solubility
Insoluble in water. Soluble in organic solvents.
Notes
Moisture sensitive. Store away from strong oxidizing agents, strong acids, bases, water/ moisture. Store under inert gas.
RUO – Research Use Only
General References:
- Miki Murata; Masanori Ishikura; Masayuki Nagata; Shinji Watanabe; and Yuzuru Masuda. Rhodium(I)-Catalyzed Silylation of Aryl Halides with Triethoxysilane: Practical Synthetic Route to Aryltriethoxysilanes. Org. Lett. 2002, 4 (11), 1843-1845.
- Amy S. Manoso and Philip DeShong. Improved Synthesis of Aryltriethoxysilanes via Palladium(0)-Catalyzed Silylation of Aryl Iodides and Bromides with Triethoxysilane. J. Org. Chem. 2001, 66 (22), 7449-7455.
- Reduziert Aldehyde und Ketone zu Alkoholen (über den Silylether) in Anwesenheit von KF oder CsF: Tetrahedron, 37, 2165 (1981). Mit CsF können Enone selektiv auf allylische Alkohole mit hoher Ausbeute reduziert werden: Tetrahedron., 39, 999 (1983).
- Vielseitiges Hydrosilylierungsmittel. Zur Verwendung in Kombination mit Ti(O-i-Pr)4 in einem katalytischen System zur Reduktion von Estern auf Alkohole siehe: J. Org. Chem., 57, 3751 (1992). VORSICHT! In dieser Reaktion kann sich stark pyrophorisches SiH4 bilden: J. Org. Chem., 57, 3221 (1993).
- Das gleiche System wirkt sich auf die ansonsten schwierige Reduktion von Phosphinoxiden zu Phosphinen aus. Die Produkte können in situ mit Alkylhalogeniden reagiert werden, um eine bequeme Eintopf-Umwandlung von Phosphinoxiden in Phosphoniumsalze zu ermöglichen: Tetrahedron Lett., 35, 625 (1994).
- Pd(0)-katalysierte Silylierung von Aryliodiden liefert einen Weg zu Aryltriethoxysilanen: J. Org. Chem., 62, 8569 (1997).