Search Thermo Fisher Scientific
Thermo Scientific Chemicals
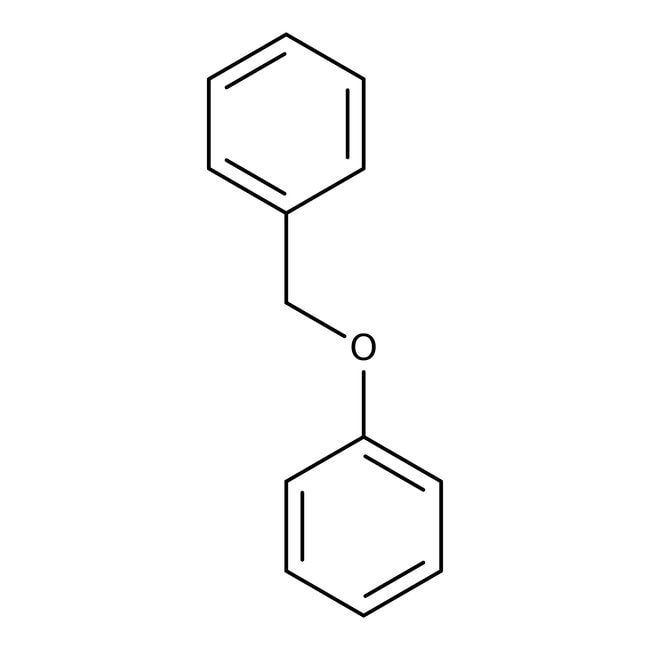
Benzyl phenyl ether, 97%, Thermo Scientific Chemicals
Catalog number B22539.06
also known as B22539-06
Price (USD)/ Each
30.80
-
Quantity:
5 g
Price (USD)/ Each
30.80
Benzyl phenyl ether, 97%, Thermo Scientific Chemicals
Catalog numberB22539.06
Price (USD)/ Each
30.80
-
Chemical Identifiers
CAS946-80-5
Specifications Specification Sheet
Specification Sheet
FormCrystals or powder or crystalline powder or fused solid
Appearance (Color)White to pale cream or pink
Assay (GC)≥96.0%
Identification (FTIR)Conforms
Melting Point (clear melt)34.0-44.0?C
Benzyl phenyl ether reacts with aluminum bromide in chlorobenzene solution to afford a mixture of phenol, o-benzyl phenol and dichlorodiphenylmethane. It is a useful as model compound in catalytic chemistry to represent the a-O4 ether bond in lignin and coal. It contains a weak ether bond of 234kJ/mol and belongs to the most thermo-labile compounds in lignin and low rank coal. Influence of alkali carbonates, common additives in biomass conversion, on the reaction pathways of BPE in superheated water has been reported. Cesium-exchanged heteropolyacid catalyzed decomposition of benzyl phenyl ether to aromatics has been investigated. Photo-Claisen rearrangements of benzyl phenyl ether was investigated in cation-exchanged Y zeolites and polyethylenes of differing crystallinities.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Benzyl phenyl ether reacts with aluminum bromide in chlorobenzene solution to afford a mixture of phenol, o-benzyl phenol and dichlorodiphenylmethane. It is a useful as model compound in catalytic chemistry to represent the a-O4 ether bond in lignin and coal. It contains a weak ether bond of 234kJ/mol and belongs to the most thermo-labile compounds in lignin and low rank coal. Influence of alkali carbonates, common additives in biomass conversion, on the reaction pathways of BPE in superheated water has been reported. Cesium-exchanged heteropolyacid catalyzed decomposition of benzyl phenyl ether to aromatics has been investigated. Photo-Claisen rearrangements of benzyl phenyl ether was investigated in cation-exchanged Y zeolites and polyethylenes of differing crystallinities.
Solubility
Insoluble in water.
Notes
Stable under recommended storage conditions. Incompatible with oxidizing agents.
Benzyl phenyl ether reacts with aluminum bromide in chlorobenzene solution to afford a mixture of phenol, o-benzyl phenol and dichlorodiphenylmethane. It is a useful as model compound in catalytic chemistry to represent the a-O4 ether bond in lignin and coal. It contains a weak ether bond of 234kJ/mol and belongs to the most thermo-labile compounds in lignin and low rank coal. Influence of alkali carbonates, common additives in biomass conversion, on the reaction pathways of BPE in superheated water has been reported. Cesium-exchanged heteropolyacid catalyzed decomposition of benzyl phenyl ether to aromatics has been investigated. Photo-Claisen rearrangements of benzyl phenyl ether was investigated in cation-exchanged Y zeolites and polyethylenes of differing crystallinities.
Solubility
Insoluble in water.
Notes
Stable under recommended storage conditions. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Richard H.Schlosberg; William H.Davis Jr.; Terrence R.Ashe. Pyrolysis studies of organic oxygenates. 2. Benzyl phenyl ether pyrolysis under batch autoclave conditions. Fuel. 1981, 60,(3), 201-204.
- Virginia Roberts; Sebastian Fendt; Angeliki A.Lemonidou; Xuebing Li; Johannes A.Lercher. Influence of alkali carbonates on benzyl phenyl ether cleavage pathways in superheated water. Applied Catalysis B: Environmental. 2010, 95,(1-2), 71-77.