Search Thermo Fisher Scientific
Thermo Scientific Chemicals
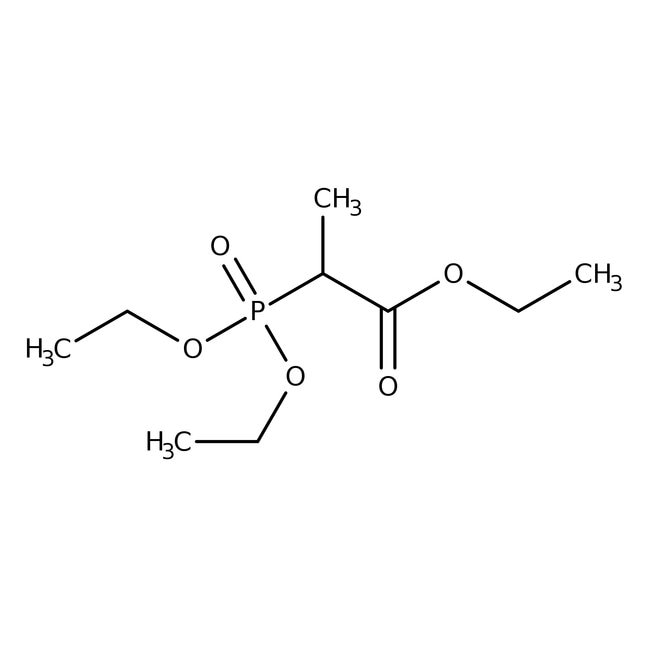
Triethyl 2-phosphonopropionate, 98%, Thermo Scientific Chemicals
Catalog number B23261.18
also known as B23261-18
Price (USD)/ Each
130.65
Online exclusive
145.00 Save 14.35 (10%)
-
Quantity:
50 g
Price (USD)/ Each
130.65
Online exclusive
145.00 Save 14.35 (10%)
Triethyl 2-phosphonopropionate, 98%, Thermo Scientific Chemicals
Catalog numberB23261.18
Price (USD)/ Each
130.65
Online exclusive
145.00 Save 14.35 (10%)
-
Chemical Identifiers
CAS3699-66-9
IUPAC Nameethyl 2-(diethoxyphosphoryl)propanoate
Molecular FormulaC9H19O5P
InChI KeyBVSRWCMAJISCTD-UHFFFAOYNA-N
SMILESCCOC(=O)C(C)P(=O)(OCC)OCC
View more
Specifications Specification Sheet
Specification Sheet
Identification (FTIR)Conforms
Refractive Index1.4295-1.4335 @ 20?C
Appearance (Color)Clear colorless
FormLiquid
Assay (GC)≥97.5%
Triethyl 2-phosphonopropionate is used as a reactant in intramolecular conjugate addition for the synthesis of floresolide B, enantioselective synthesis of spiroindane di-methyl acetic acid and in stereo selective intramolecular Diels-Alder reactions. It plays an important role in Horner-Wadsworth-Emmons reactions and chemoenzymatic one-pot synthesis of gamma-butyrolactones. It is also used in the preparation of 3-[2]furyl-2-methyl-acrylic acid ethyl ester by reacting with furfural.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Triethyl 2-phosphonopropionate is used as a reactant in intramolecular conjugate addition for the synthesis of floresolide B, enantioselective synthesis of spiroindane di-methyl acetic acid and in stereo selective intramolecular Diels-Alder reactions. It plays an important role in Horner-Wadsworth-Emmons reactions and chemoenzymatic one-pot synthesis of gamma-butyrolactones. It is also used in the preparation of 3-[2]furyl-2-methyl-acrylic acid ethyl ester by reacting with furfural.
Solubility
Miscible with chloroform and methanol.
Notes
Incompatible with strong bases.
Triethyl 2-phosphonopropionate is used as a reactant in intramolecular conjugate addition for the synthesis of floresolide B, enantioselective synthesis of spiroindane di-methyl acetic acid and in stereo selective intramolecular Diels-Alder reactions. It plays an important role in Horner-Wadsworth-Emmons reactions and chemoenzymatic one-pot synthesis of gamma-butyrolactones. It is also used in the preparation of 3-[2]furyl-2-methyl-acrylic acid ethyl ester by reacting with furfural.
Solubility
Miscible with chloroform and methanol.
Notes
Incompatible with strong bases.
RUO – Research Use Only
General References:
- Wadsworth-Emmons precursor of methacrylic esters (see Triethyl phosphonoacetate, A14120 and Appendix 1). Lithiation followed by acylation with perfluoroalkanoic anhydrides gives a perfluoroacyl intermediate which, with an organolithium reagent, leads stereoselectively to a trifluoroalkylated ɑß-unsaturated ester: J. Fluorine Chem., 89, 141 (1998):
- Sharpe, M. A.; Han, J.; Baskin, A. M.; Baskin, D. S. Design and Synthesis of a MAO-B-Selectively Activated Prodrug Based on MPTP: A Mitochondria-Targeting Chemotherapeutic Agent for Treatment of Human Malignant Gliomas. ChemMedChem 2015, 10 (4), 621-628.
- Shouksmith, A. E.; Evans, L. E.; Tweddle, D. A.; Miller, D. C.; Willmore, E.; Newell, D. R.; Golding, B. T.; Griffin, R. J. Synthesis and Activity of Putative Small-molecule Inhibitors of the F-box Protein SKP2. Aust. J. Chem. 2015, 68 (4), 660-679.