Search Thermo Fisher Scientific
Thermo Scientific Chemicals
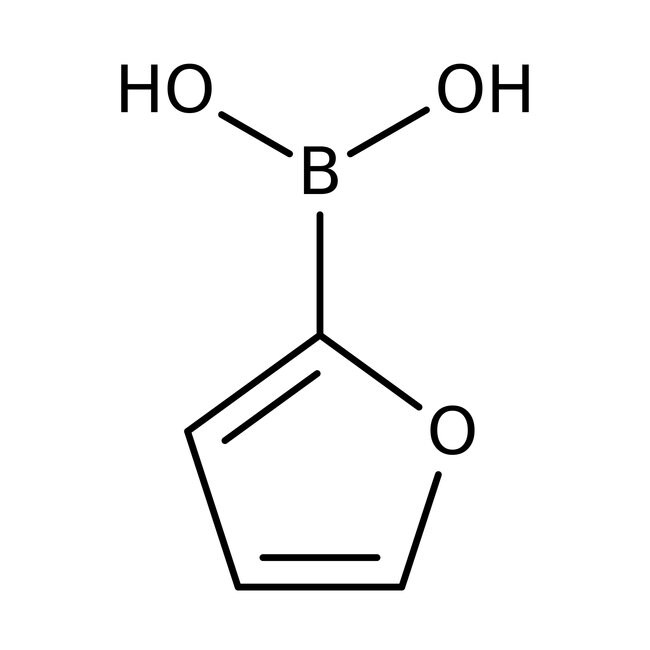
Furan-2-boronic acid, 97%, Thermo Scientific Chemicals
Catalog number B23842.06
also known as B23842-06
Price (USD)/ Each
180.65
Online exclusive
200.00 Save 19.35 (10%)
-
Quantity:
5 g
Price (USD)/ Each
180.65
Online exclusive
200.00 Save 19.35 (10%)
Furan-2-boronic acid, 97%, Thermo Scientific Chemicals
Catalog numberB23842.06
Price (USD)/ Each
180.65
Online exclusive
200.00 Save 19.35 (10%)
-
Chemical Identifiers
CAS13331-23-2
IUPAC Name(furan-2-yl)boronic acid
Molecular FormulaC4H5BO3
InChI KeyPZJSZBJLOWMDRG-UHFFFAOYSA-N
SMILESOB(O)C1=CC=CO1
View more
Specifications Specification Sheet
Specification Sheet
FormCrystals or powder or crystalline powder
Assay (Aqueous acid-base Titration)≥96.0%
Assay (HPLC)≥96.0%
Proton NMRConforms to structure
Appearance (Color)Pale cream to cream
It is used in Suzuki reaction for generalized route for the synthesis of β-furyl-α,β-unsaturated aldehydes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is used in Suzuki reaction for generalized route for the synthesis of β-furyl-α,β-unsaturated aldehydes.
Solubility
Slightly soluble in water.
Notes
Air sensitive. Store away from oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
It is used in Suzuki reaction for generalized route for the synthesis of β-furyl-α,β-unsaturated aldehydes.
Solubility
Slightly soluble in water.
Notes
Air sensitive. Store away from oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
RUO – Research Use Only
General References:
- Michael J. Burns, et al. Simple Palladium(II) Precatalyst for Suzuki-Miyaura Couplings: Efficient Reactions of Benzylic, Aryl, Heteroaryl, and Vinyl Coupling Partners.Org. Lett.,2007,9(26), 5397-5400.
- Khokan Samanta, et al. A generalized route for the synthesis of β-furyl-α,β-unsaturated aldehydes through Suzuki reactions.Tetrahedron Lett.,2008,49(9), 1461-1464.
- Undergoes an asymmetric boronic acid Mannich reaction with aldehydes in the presence of the chiral template (S)-5-phenylmorpholine-2-one to give substituted furfurylamines with high diastereomeric excess: Chem. Commun., 1953 (1996); J. Chem. Soc., Perkin 1, 2982 (2000):