Search Thermo Fisher Scientific
Thermo Scientific Chemicals
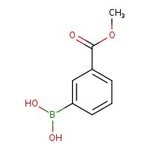
3-(Methoxycarbonyl)benzeneboronic acid, 97%, Thermo Scientific Chemicals
Catalog number: H27444.03
1 g, Each


Thermo Scientific Chemicals
3-(Methoxycarbonyl)benzeneboronic acid, 97%, Thermo Scientific Chemicals
Catalog number: H27444.03
1 g, Each
Quantity
Catalog number: H27444.03
also known as H27444-03
Price (USD)
58.30
Each
Quantity
-
Have Questions?
Chemical Identifiers
CAS
99769-19-4
IUPAC Name
[3-(methoxycarbonyl)phenyl]boronic acid
Molecular Formula
C8H9BO4
InChI Key
ALTLCJHSJMGSLT-UHFFFAOYSA-N
SMILES
COC(=O)C1=CC=CC(=C1)B(O)O
Specifications
Form
Powder
Assay (Aqueous acid-base Titration)
≥96.0%
Proton NMR
Conforms to structure
Appearance (Color)
White to pale cream
Assay (HPLC)
≥96.0%
Description
Reagent used for tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence, copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides, one-pot ipso-nitration of arylboronic acids, copper-catalyzed nitration, cyclocondensation followed by palladium-phosphine-catalyzed Suzuki-Miyaura coupling. Reagent used in Preparation of biaryls via nickel-catalyzed Suzuki-Miyaura cross-coupling reaction of aryl halides with arylboronic acid6, chromenones and their bradykinin B1 antagonistic active, Pt nanoparticles at Photoactive metal-organic frameworks resulting in efficient hydrogen evolution via synergistic photoexcitation and electron injecti, salicylate-based thienylbenzoic acids as E. coli methionine aminopeptidase inhibitor.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Reagent used for tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence, copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides, one-pot ipso-nitration of arylboronic acids, copper-catalyzed nitration, cyclocondensation followed by palladium-phosphine-catalyzed Suzuki-Miyaura coupling. Reagent used in Preparation of biaryls via nickel-catalyzed Suzuki-Miyaura cross-coupling reaction of aryl halides with arylboronic acid6, chromenones and their bradykinin B1 antagonistic active, Pt nanoparticles at Photoactive metal-organic frameworks resulting in efficient hydrogen evolution via synergistic photoexcitation and electron injecti, salicylate-based thienylbenzoic acids as E. coli methionine aminopeptidase inhibitor.
Solubility
Reacts with water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents.
Reagent used for tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence, copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides, one-pot ipso-nitration of arylboronic acids, copper-catalyzed nitration, cyclocondensation followed by palladium-phosphine-catalyzed Suzuki-Miyaura coupling. Reagent used in Preparation of biaryls via nickel-catalyzed Suzuki-Miyaura cross-coupling reaction of aryl halides with arylboronic acid6, chromenones and their bradykinin B1 antagonistic active, Pt nanoparticles at Photoactive metal-organic frameworks resulting in efficient hydrogen evolution via synergistic photoexcitation and electron injecti, salicylate-based thienylbenzoic acids as E. coli methionine aminopeptidase inhibitor.
Solubility
Reacts with water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text