Search Thermo Fisher Scientific
Thermo Scientific Chemicals
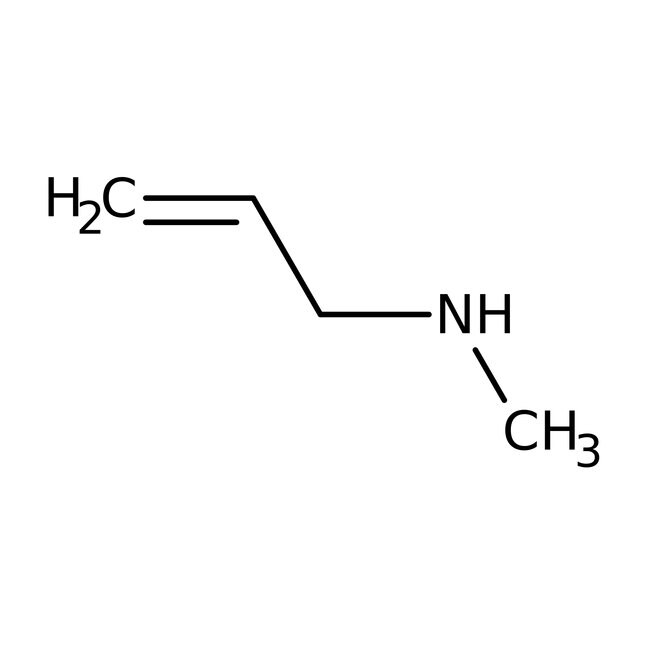
N-Allylmethylamine, 96%, Thermo Scientific Chemicals
Catalog number H27551.06
also known as H27551-06
Price (USD)/ Each
200.65
Online exclusive
223.00 Save 22.35 (10%)
-
Quantity:
5 g
Price (USD)/ Each
200.65
Online exclusive
223.00 Save 22.35 (10%)
N-Allylmethylamine, 96%, Thermo Scientific Chemicals
Catalog numberH27551.06
Price (USD)/ Each
200.65
Online exclusive
223.00 Save 22.35 (10%)
-
Chemical Identifiers
CAS627-37-2
IUPAC Namemethyl(prop-2-en-1-yl)amine
Molecular FormulaC4H9N
InChI KeyIOXXVNYDGIXMIP-UHFFFAOYSA-N
SMILESCNCC=C
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to yellow
Refractive Index1.4095-1.4155 @ 20?C
FormLiquid
Assay (GC)≥95.0%
Identification (FTIR)Conforms
N-Allylmethylamine is used in the production of N-allyl-alfa,alfa-dichloro-N-methylacetamide. It is used as a functional monomer to coordinate with Cd(II)/Zn(II) ions to get the poly methyl methacrylate -butyl methacrylate - N-allylmethylamine (PMBA) films, which is useful as building blocks to fabricate a photo-catalytic cell.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
N-Allylmethylamine is used in the production of N-allyl-alfa,alfa-dichloro-N-methylacetamide. It is used as a functional monomer to coordinate with Cd(II)/Zn(II) ions to get the poly methyl methacrylate -butyl methacrylate - N-allylmethylamine (PMBA) films, which is useful as building blocks to fabricate a photo-catalytic cell.
Solubility
Miscible with water.
Notes
Store in a cool place. Incompatible with oxidizing agents, strong acids and bases.
N-Allylmethylamine is used in the production of N-allyl-alfa,alfa-dichloro-N-methylacetamide. It is used as a functional monomer to coordinate with Cd(II)/Zn(II) ions to get the poly methyl methacrylate -butyl methacrylate - N-allylmethylamine (PMBA) films, which is useful as building blocks to fabricate a photo-catalytic cell.
Solubility
Miscible with water.
Notes
Store in a cool place. Incompatible with oxidizing agents, strong acids and bases.
RUO – Research Use Only
General References:
- Gibbons, J. B.; Salvant, J. M.; Vaden, R. M.; Kwon, K. H.; Welm, B. E.; Looper, R. E. Synthesis of Naamidine A and Selective Access to N2-Acyl-2-aminoimidazole Analogues. J. Org. Chem. 2015, 80 (20), 10076-10085.
- Jaganathan, S. K.; Balaji, A.; Vellayappan, M. V.; Subramanian, A. P.; John, A. A.; Asokan, M. K.; Supriyanto, E. Review: Radiation-induced surface modification of polymers for biomaterial application. J. Mater. Sci. 2015, 50 (5), 2007-2018.