Search Thermo Fisher Scientific
Thermo Scientific Chemicals
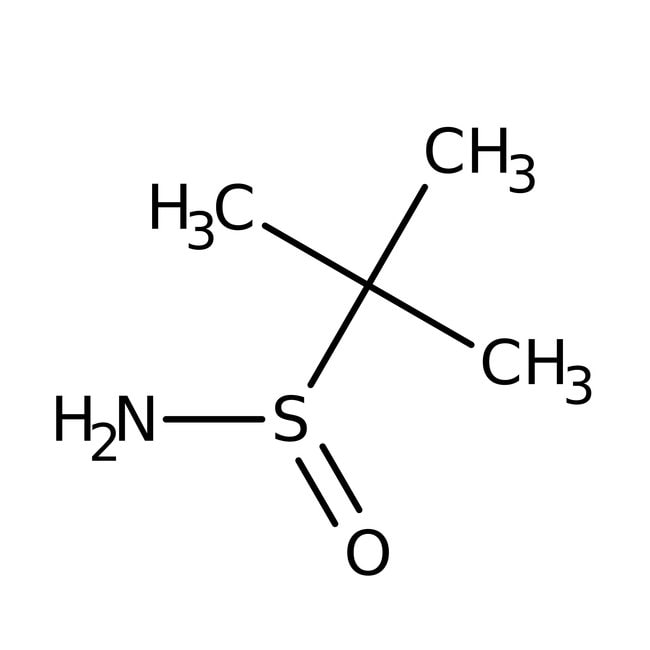
(R)-(+)-2-Methyl-2-propanesulfinamide, 98%, Thermo Scientific Chemicals
Catalog number: H27724.03
1 g, Each

Thermo Scientific Chemicals
(R)-(+)-2-Methyl-2-propanesulfinamide, 98%, Thermo Scientific Chemicals
Catalog number: H27724.03
1 g, Each
Quantity
Catalog number: H27724.03
also known as H27724-03
Price (USD)
69.00
Each
Quantity
-
Have Questions?
Chemical Identifiers
CAS
196929-78-9
IUPAC Name
2-methylpropane-2-sulfinamide
Molecular Formula
C4H11NOS
InChI Key
CESUXLKAADQNTB-UHFFFAOYNA-N
SMILES
CC(C)(C)S(N)=O
Specifications
Appearance (Color)
White to pale cream
Assay (GC)
≥97.5%
Form
Crystals or powder or crystalline powder
Optical Rotation
5.0 ± 1.5? (C=1 in Chloroform)
Description
(R)-(+)-2-Methyl-2-propanesulfinamide is a chiral ligand, which is used in pharmaceutical compositions. Further, it is used in the preparation of beta-chloro sulfinamides in the synthesis of chiral azridines. It is involved in the preparation of organocatalyst for enantioselective reduction of imines. It serves as a reagent for synthesizing chiral amines. In addition to this, it is converted into P,N-sulfinyl imine ligands through condensation with aldehydes and ketones which undergoes iridium-catalyzed asymmetric hydrogenation of olefins.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
(R)-(+)-2-Methyl-2-propanesulfinamide is a chiral ligand, which is used in pharmaceutical compositions. Further, it is used in the preparation of beta-chloro sulfinamides in the synthesis of chiral azridines. It is involved in the preparation of organocatalyst for enantioselective reduction of imines. It serves as a reagent for synthesizing chiral amines. In addition to this, it is converted into P,N-sulfinyl imine ligands through condensation with aldehydes and ketones which undergoes iridium-catalyzed asymmetric hydrogenation of olefins.
Solubility
Soluble in chloroform, methanol, tetrahydrofuran, dichloromethane, dimethyl sulfoxide and most organic solvents.
Notes
Incompatible with strong oxidizing agents, strong acids and bases. Store in a cool place.
(R)-(+)-2-Methyl-2-propanesulfinamide is a chiral ligand, which is used in pharmaceutical compositions. Further, it is used in the preparation of beta-chloro sulfinamides in the synthesis of chiral azridines. It is involved in the preparation of organocatalyst for enantioselective reduction of imines. It serves as a reagent for synthesizing chiral amines. In addition to this, it is converted into P,N-sulfinyl imine ligands through condensation with aldehydes and ketones which undergoes iridium-catalyzed asymmetric hydrogenation of olefins.
Solubility
Soluble in chloroform, methanol, tetrahydrofuran, dichloromethane, dimethyl sulfoxide and most organic solvents.
Notes
Incompatible with strong oxidizing agents, strong acids and bases. Store in a cool place.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text