Search Thermo Fisher Scientific
Thermo Scientific Chemicals
4-Fluorobenzoic anhydride, 97%, Thermo Scientific Chemicals
Catalog number H33527.06
also known as H33527-06
Price (USD)/ Each
292.00
-
Quantity:
5 g
Price (USD)/ Each
292.00
4-Fluorobenzoic anhydride, 97%, Thermo Scientific Chemicals
Catalog numberH33527.06
Price (USD)/ Each
292.00
-
Chemical Identifiers
CAS25569-77-1
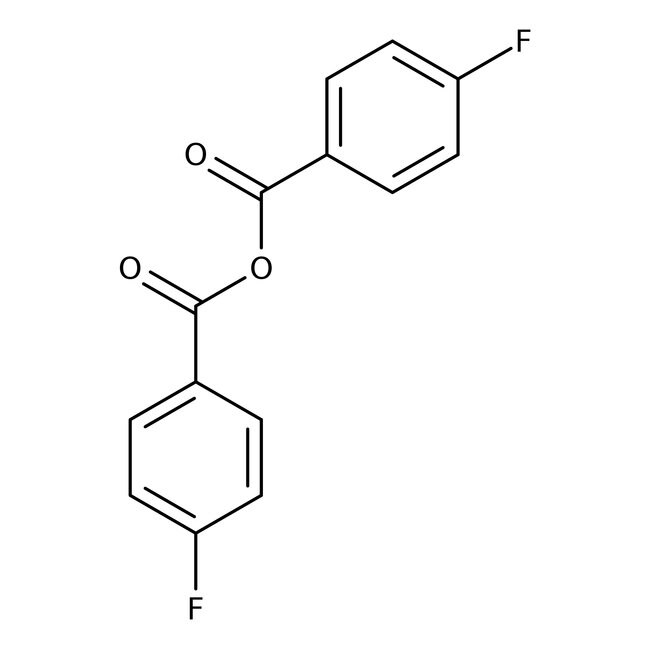
IUPAC Name4-fluorobenzoyl 4-fluorobenzoate
Molecular FormulaC14H8F2O3
InChI KeyBBLXFRIGTQYGOT-UHFFFAOYSA-N
SMILESFC1=CC=C(C=C1)C(=O)OC(=O)C1=CC=C(F)C=C1
View more
Specifications Specification Sheet
Specification Sheet
Melting Point (clear melt)109.5-118.5?C
Appearance (Color)White
Assay (GC)≥96.0%
FormCrystals or powder or crystalline powder
It is used as pharmaceutical intermediate. Benzoylthiophenes are allosteric enhancers (AE) of agonist activity at the A1 adenosine receptor.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is used as pharmaceutical intermediate. Benzoylthiophenes are allosteric enhancers (AE) of agonist activity at the A1 adenosine receptor.
Solubility
Sparingly soluble in water.(0.26 g/L) (25°C),
Notes
Store in cool dry place. Ensure proper ventilation. Incompatible with oxidizing agents.
It is used as pharmaceutical intermediate. Benzoylthiophenes are allosteric enhancers (AE) of agonist activity at the A1 adenosine receptor.
Solubility
Sparingly soluble in water.(0.26 g/L) (25°C),
Notes
Store in cool dry place. Ensure proper ventilation. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- C. Elisabet Tranberg.; Andrea Zickgraf.; Brian N. Giunta.; Henning Luetjens.; Heidi Figler.; Lauren J. Murphree.; Ruediger Falke.; Holger Fleischer.; Joel Linden.; Peter J. Scammells.; Ray. A. Olsson. 2-Amino-3-aroyl-4,5-alkylthiophenes: Agonist Allosteric Enhancers at Human A1 Adenosine Receptors.J. Med. Chem. 2002, 45 (2),382-389 .
- Robert H. Dodd.; Catherine Ouannes.; Malka Robert-Gero.; Pierre Potier. Hybrid molecules: growth inhibition of Leishmania donovani promastigotes by thiosemicarbazones of 3-carboxy-.beta.-carbolines.J. Med. Chem. 1989, 32 (6),1272-1276 .