Search Thermo Fisher Scientific
Thermo Scientific Chemicals
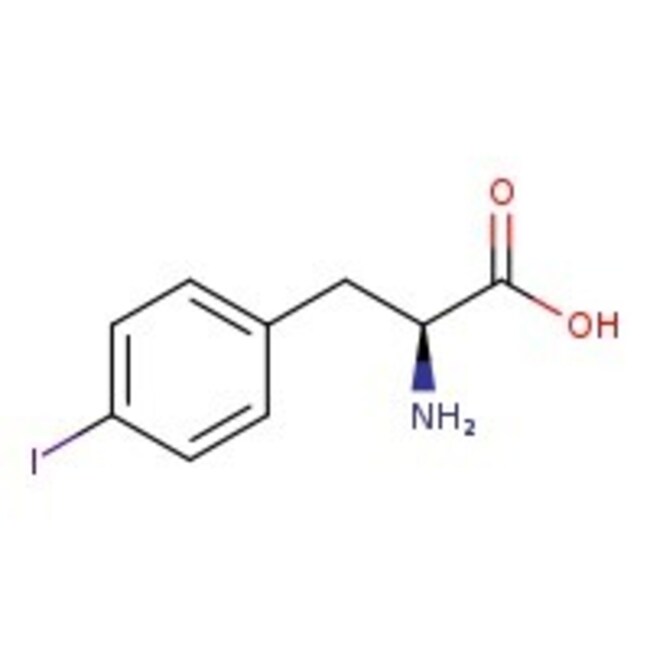
4-Iodo-L-phenylalanine, 95%, Thermo Scientific Chemicals
Catalog number H51979.06
also known as H51979-06
Price (USD)/ Each
380.00
-
Quantity:
5 g
Price (USD)/ Each
380.00
4-Iodo-L-phenylalanine, 95%, Thermo Scientific Chemicals
Catalog numberH51979.06
Price (USD)/ Each
380.00
-
Chemical Identifiers
CAS24250-85-9
IUPAC Name(2S)-2-amino-3-(4-iodophenyl)propanoic acid
Molecular FormulaC9H10INO2
InChI KeyPZNQZSRPDOEBMS-QMMMGPOBSA-N
SMILESN[C@@H](CC1=CC=C(I)C=C1)C(O)=O
View more
Specifications Specification Sheet
Specification Sheet
Assay (unspecified)>94.0%
4-Iodo-L-phenylalanine may be used in protein engineering as a model unnatural α amino acid to alter primary amino acid composition via the opal (UGA) codon.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Iodo-L-phenylalanine may be used in protein engineering as a model unnatural α amino acid to alter primary amino acid composition via the opal (UGA) codon.
Solubility
Partly miscible with water.
Notes
Light sensitive. Incompatible with oxidizing agents.
4-Iodo-L-phenylalanine may be used in protein engineering as a model unnatural α amino acid to alter primary amino acid composition via the opal (UGA) codon.
Solubility
Partly miscible with water.
Notes
Light sensitive. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Koichiro Kodama; Dr. Seketsu Fukuzawa; Dr. Kensaku Sakamoto; Dr. Hiroshi Nakayama; Prof. Takanori Kigawa; Takashi Yabuki; Natsuko Matsuda; Dr. Mikako Shirouzu; Dr. Koji Takio; Kazuo Tachibana; Prof. Shigeyuki Yokoyama. A new protein engineering approach combining chemistry and biology, Part I; site-specific incorporation of 4-iodo-L-phenylalanine in vitro by using misacylated suppressor tRNAPhe . ChemBioChem. 2006, 7, (10),1577-1581
- Enrico Morera; Giorgio Ortar. A concise synthesis of photoactivatable 4-Aroyl-l-phenylalanines. Bioorganic & Medicinal Chemistry Letters. 2000, 10, (16),1815-1818