Search Thermo Fisher Scientific
Thermo Scientific Chemicals
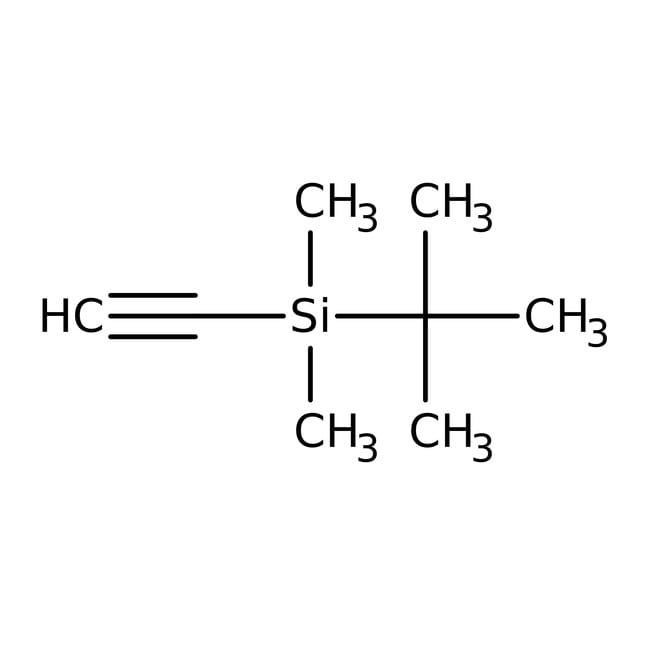
tert-Butyldimethylsilylacetylene, 98%, Thermo Scientific Chemicals
Catalog number H53494.03
also known as H53494-03
Price (USD)/ Each
71.80
-
Quantity:
1 g
Price (USD)/ Each
71.80
tert-Butyldimethylsilylacetylene, 98%, Thermo Scientific Chemicals
Catalog numberH53494.03
Price (USD)/ Each
71.80
-
Specifications
Boiling Point130°C
Chemical Name or Materialtert-Butyldimethylsilylacetylene
CAS86318-61-8
Health Hazard 1H225-H315-H319-H335
Health Hazard 2GHS H Statement
H225-H315-H319-H335
Highly flammable liquid and vapor.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H225-H315-H319-H335
Highly flammable liquid and vapor.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
View more
tert-Butyldimethylsilylacetylene may be used in the synthesis of β-alkynylketone and β-alkynyl aldehydes. It participates in Cadiot-Chodkiewicz cross-coupling reaction with various bromoalkynes to afford synthetically useful unsymmetrical diynes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
tert-Butyldimethylsilylacetylene may be used in the synthesis of β-alkynylketone and β-alkynyl aldehydes. It participates in Cadiot-Chodkiewicz cross-coupling reaction with various bromoalkynes to afford synthetically useful unsymmetrical diynes.
Solubility
Sparingly soluble in water (0.043 g/L at 25°C).
Notes
Stable under recommended storage conditions.
tert-Butyldimethylsilylacetylene may be used in the synthesis of β-alkynylketone and β-alkynyl aldehydes. It participates in Cadiot-Chodkiewicz cross-coupling reaction with various bromoalkynes to afford synthetically useful unsymmetrical diynes.
Solubility
Sparingly soluble in water (0.043 g/L at 25°C).
Notes
Stable under recommended storage conditions.
RUO – Research Use Only
General References:
- Dr. Ryo Shintani; Keishi Takatsu, Taisuke Katoh, Dr. Takahiro Nishimura; Prof. Dr. Tamio Hayashi. Rhodium-catalyzed rearrangement of aryl bis(alkynyl) carbinols to 3-alkynyl-1-indanones . Angewandte Chemie. 2008, 120, (8),1469-1471
- Dr. Takahiro Nishimura; Takahiro Sawano; Prof. Tamio Hayashi. Asymmetric synthesis of β-alkynyl aldehydes by rhodium-catalyzed conjugate alkynylation. Angewandte Chemie. 2009, 121, (43),8201-8203