Search Thermo Fisher Scientific
Thermo Scientific Chemicals
2-(Chloromethoxy)ethyltrimethylsilane, tech. 90%, stab. with 0.1% N,N-Diisopropylethylamine, Thermo Scientific Chemicals
Catalog number H55074.03
also known as H55074-03
Price (USD)/ Each
42.90
-
Quantity:
1 g
Price (USD)/ Each
42.90
2-(Chloromethoxy)ethyltrimethylsilane, tech. 90%, stab. with 0.1% N,N-Diisopropylethylamine, Thermo Scientific Chemicals
Catalog numberH55074.03
Price (USD)/ Each
42.90
-
Chemical Identifiers
CAS76513-69-4
IUPAC Name[2-(chloromethoxy)ethyl]trimethylsilane
Molecular FormulaC6H15ClOSi
InChI KeyBPXKZEMBEZGUAH-UHFFFAOYSA-N
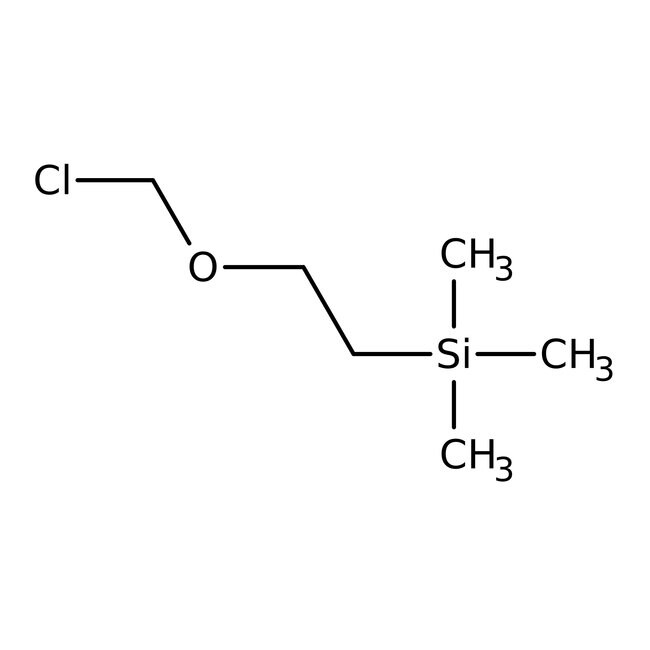
SMILESC[Si](C)(C)CCOCCl
View more
2-(Chloromethoxy)ethyltrimethylsilane is used in the preparation of SEM [2-(trimethylsilyl)ethoxy]methyl]-ethers. It serves as a phenol protecting group in the synthesis of laterifluorones. Further, it reacts with 1H-imidazole to prepare 1-(2-trimethylsilanyl-ethoxymethyl)-1H-imidazole.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-(Chloromethoxy)ethyltrimethylsilane is used in the preparation of SEM [2-(trimethylsilyl)ethoxy]methyl]-ethers. It serves as a phenol protecting group in the synthesis of laterifluorones. Further, it reacts with 1H-imidazole to prepare 1-(2-trimethylsilanyl-ethoxymethyl)-1H-imidazole.
Notes
Store in a cool place. Moisture sensitive. Incompatible with strong oxidizing agents.
2-(Chloromethoxy)ethyltrimethylsilane is used in the preparation of SEM [2-(trimethylsilyl)ethoxy]methyl]-ethers. It serves as a phenol protecting group in the synthesis of laterifluorones. Further, it reacts with 1H-imidazole to prepare 1-(2-trimethylsilanyl-ethoxymethyl)-1H-imidazole.
Notes
Store in a cool place. Moisture sensitive. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Saito, A.; Higgins, M.; Zheng, S.; Li, W.; Ojima, I.; Dinkova-Kostova, A. T.; Honda, T. Synthesis and biological evaluation of biotin conjugates of (±)-(4bS,8aR,10aS)-10a-ethynyl-4b,8,8-trimethyl-3,7-dioxo-3,4b,7,8,8a,9,10,10a-octahydro-phenanthrene-2,6-dicarbonitrile, an activator of the Keap1rf2/ARE pathway, for the isolation of its protein targets. Bioorg. Med. Chem. Lett. 2013, 23 (20), 5540-5543.
- Gonzalez, A. Z.; Li, Z.; Beck, H. P.; Canon, J.; Chen, A.; Chow, D.; Duquette, J.; Eksterowicz, J.; Fox, B. M.; Fu, J.; Huang, X.; Houze, J.; Jin, L.; Li, Y.; Ling, Y.; Lo, M. C.; Long, A. M.; McGee, L. R.; McIntosh, J.; Oliner, J. D.; Osgood, T.; Rew, Y.; Saiki, A. Y.; Shaffer, P.; Wortman, S.; Yakowec, P.; Yan, X.; Ye, Q.; Yu, D.; Zhao, X.; Zhou, J.; Olson, S. H.; Sun, D.; Medina, J. C. Novel Inhibitors of the MDM2-p53 Interaction Featuring Hydrogen Bond Acceptors as Carboxylic Acid Isosteres. J. Med. Chem. 2014, 57 (7), 2963-2988.