Search Thermo Fisher Scientific
Thermo Scientific Chemicals
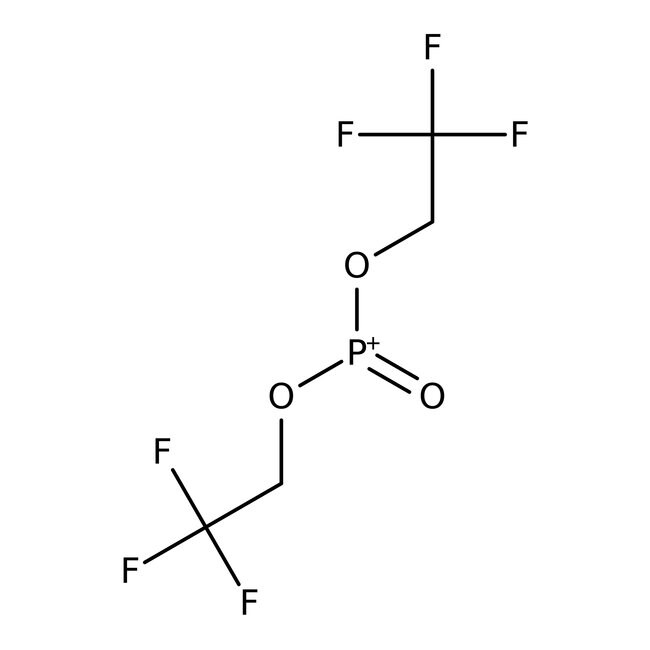
Bis(2,2,2-trifluoroethyl) phosphite, tech. 90%, Thermo Scientific Chemicals
Catalog number H55562.03
also known as H55562-03
Price (USD)/ Each
23.30
-
Quantity:
1 g
Price (USD)/ Each
23.30
Bis(2,2,2-trifluoroethyl) phosphite, tech. 90%, Thermo Scientific Chemicals
Catalog numberH55562.03
Price (USD)/ Each
23.30
-
Specifications
Chemical Name or MaterialBis(2,2,2-trifluoroethyl) phosphite
CAS92466-70-1
Health Hazard 1H227-H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335-H227
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
H315-H319-H335-H227
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
Health Hazard 3P210-P235-P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P370+P378q-P501c
View more
Bis(2,2,2-trifluoroethyl) phosphite was used in the synthesis of bis(2,2,2-trifluoroethyl phosphorochloridate. It was also employed as reagent for the synthesis of mono- and diesters of phosphorous acid.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Bis(2,2,2-trifluoroethyl) phosphite was used in the synthesis of bis(2,2,2-trifluoroethyl phosphorochloridate. It was also employed as reagent for the synthesis of mono- and diesters of phosphorous acid.
Notes
Store at room temperature. Avoid strong oxidizing agents.
Bis(2,2,2-trifluoroethyl) phosphite was used in the synthesis of bis(2,2,2-trifluoroethyl phosphorochloridate. It was also employed as reagent for the synthesis of mono- and diesters of phosphorous acid.
Notes
Store at room temperature. Avoid strong oxidizing agents.
RUO – Research Use Only
General References:
- Guy D. Joly.; Eric N. Jacobsen. Thiourea-Catalyzed Enantioselective Hydrophosphonylation of Imines: Practical Access to Enantiomerically Enriched α-Amino Phosphonic Acids. J. Am. Chem. Soc. 2004, 126 (13),4102-4103.
- Richard J Cohen.; Daniel L Fox.; Jarrod F Eubank.; Ralph Nicholas Salvatore. Mild and efficient Cs2CO3-promoted synthesis of phosphonates. Tetrahedron Lett. 2003, 44 (47),8617-8621.