Search Thermo Fisher Scientific
Thermo Scientific Chemicals
Doxorubicin hydrochloride, 10 mg/ml in dH2O, sterilised, Thermo Scientific Chemicals
Catalog number: J67209.XF
1 mL, Each


Thermo Scientific Chemicals
Doxorubicin hydrochloride, 10 mg/ml in dH2O, sterilised, Thermo Scientific Chemicals
Catalog number: J67209.XF
1 mL, Each
Quantity
Catalog number: J67209.XF
also known as J67209-XF
Price (USD)
544.00
Each
Quantity
-
Have Questions?
Chemical Identifiers
CAS
25316-40-9
IUPAC Name
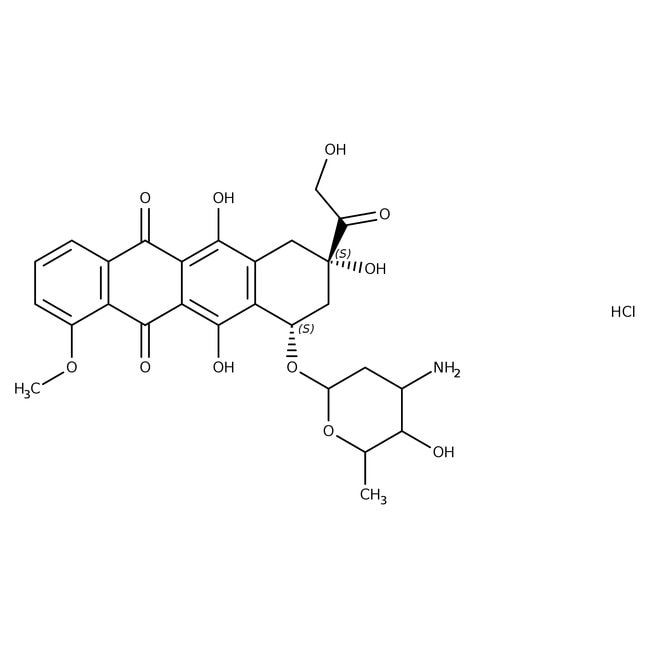
hydrogen (8S,10S)-10-[(4-amino-5-hydroxy-6-methyloxan-2-yl)oxy]-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-5,7,8,9,10,12-hexahydrotetracene-5,12-dione chloride
Molecular Formula
C27H30ClNO11
InChI Key
MWWSFMDVAYGXBV-FGBJBKNOSA-N
SMILES
[H+].[Cl-].COC1=CC=CC2=C1C(=O)C1=C(O)C3=C(C[C@](O)(C[C@@H]3OC3CC(N)C(O)C(C)O3)C(=O)CO)C(O)=C1C2=O
Specifications
Form
Liquid
Composition
Doxorubicin hydrochloride: 10 mg/mL
Description
Doxorubicin hydrochloride is a DNA intercalator and powerful cytotoxic agent. Doxorubicin inhibits topoisomerase II which results in an increased and stabilized cleavable enzyme-DNA linked complex during DNA replication and subsequently prevents the ligation of the nucleotide strand after double-strand breakage.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
General Description
• Doxorubicin hydrochloride, isolated from the bacterium Streptomyces peucetius, is an anthracycline antibiotic with antineoplastic activity and is related to daunorubicin
• It can intercalate between base pairs in the DNA helix preventing DNA replication
• It also inhibits nucleic acid synthesis and can induce apoptosis
• It can inhibit DNA topoisomerase II, resulting in an increased and stabilized cleavable enzyme-DNA linked complex during DNA replication, and prevents nucleotide strand ligation after double-strand breakage
• Doxorubicin hydrochloride forms oxygen free radicals that results in cytotoxicity and leads to the lipid peroxidation of cell membrane lipids
Application
• This compound displays a fluorescent property allowing the measurement of drug efflux pump activity and examining the role of subcellular organelles (Golgi complex and lysosome) in the sequestration of drugs
• It has been used in cell viability assays
• It is used as a drug in polymeric PLGA-based microparticulate drug delivery and to develop in vitro doxorubicin-resistant HepG2 cells (HepG2-DR) and K562 cells (K562-DR)
• It is a substrate of the MRP1 transporter
WARNING: Cancer and Reproductive Harm – www.P65Warnings.ca.gov
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text