Search Thermo Fisher Scientific
Thermo Scientific Chemicals
2-Methoxyethoxymethyl chloride, 94%, Thermo Scientific Chemicals
Catalog number: L01050.06
5 g, Each


Thermo Scientific Chemicals
2-Methoxyethoxymethyl chloride, 94%, Thermo Scientific Chemicals
Catalog number: L01050.06
5 g, Each
Quantity
Catalog number: L01050.06
also known as L01050-06
Price (USD)
52.70
Each
Quantity
-
Have Questions?
Chemical Identifiers
CAS
3970-21-6
IUPAC Name
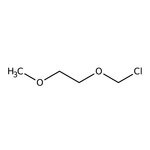
1-(chloromethoxy)-2-methoxyethane
Molecular Formula
C4H9ClO2
InChI Key
BIAAQBNMRITRDV-UHFFFAOYSA-N
SMILES
COCCOCCl
Specifications
Appearance (Color)
Clear, colorless to pale yellow
Form
Liquid
Assay (GC)
>92.5%
Refractive Index
1.4235-1.4305 @ 20?C
Description
2-Methoxyethoxymethyl chloride, is used as selectively cleaved under aprotic conditions in the presence of a wide range of OH-protected reagents. It is used as an OH-protecting reagent. An examples of the target molecule MEM Chloride is the side chain of roxithromycin. It is also used in the protection of the OH groups in serine and threonine during peptide synthesis. Some of the other applications include have the ability to coordinate to metals, which is thought to accelerate the cleavage by Lewis acids. The chelating ability of the MEM ether also makes it useful as a stereodirecting group in organometallic reactions, first noted in the stereo controlled addition of ?-methoxyvinyllithium to a carbonyl in the synthesis of taxusin.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Methoxyethoxymethyl chloride, is used as selectively cleaved under aprotic conditions in the presence of a wide range of OH-protected reagents. It is used as an OH-protecting reagent. An examples of the target molecule MEM Chloride is the side chain of roxithromycin. It is also used in the protection of the OH groups in serine and threonine during peptide synthesis. Some of the other applications include have the ability to coordinate to metals, which is thought to accelerate the cleavage by Lewis acids. The chelating ability of the MEM ether also makes it useful as a stereodirecting group in organometallic reactions, first noted in the stereo controlled addition of ɑ-methoxyvinyllithium to a carbonyl in the synthesis of taxusin.
Solubility
It hydrolyzes with water.
Notes
Moisture, Heat, water Sensitive. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Keep away from oxidizing agents. Stable under recommended storage conditions.
2-Methoxyethoxymethyl chloride, is used as selectively cleaved under aprotic conditions in the presence of a wide range of OH-protected reagents. It is used as an OH-protecting reagent. An examples of the target molecule MEM Chloride is the side chain of roxithromycin. It is also used in the protection of the OH groups in serine and threonine during peptide synthesis. Some of the other applications include have the ability to coordinate to metals, which is thought to accelerate the cleavage by Lewis acids. The chelating ability of the MEM ether also makes it useful as a stereodirecting group in organometallic reactions, first noted in the stereo controlled addition of ɑ-methoxyvinyllithium to a carbonyl in the synthesis of taxusin.
Solubility
It hydrolyzes with water.
Notes
Moisture, Heat, water Sensitive. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Keep away from oxidizing agents. Stable under recommended storage conditions.
WARNING: Cancer – www.P65Warnings.ca.gov
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text