Search Thermo Fisher Scientific
Thermo Scientific Chemicals
Benzyl isocyanate, 98%, Thermo Scientific Chemicals
Catalog number L02633.03
also known as L02633-03
Price (USD)/ Each
28.70
-
Quantity:
1 g
Price (USD)/ Each
28.70
Benzyl isocyanate, 98%, Thermo Scientific Chemicals
Catalog numberL02633.03
Price (USD)/ Each
28.70
-
Chemical Identifiers
CAS3173-56-6
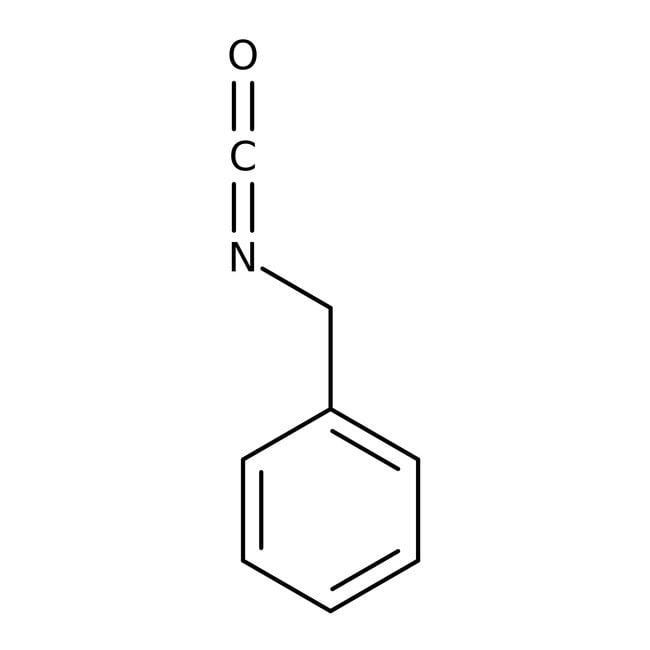
IUPAC Name(isocyanatomethyl)benzene
Molecular FormulaC8H7NO
InChI KeyYDNLNVZZTACNJX-UHFFFAOYSA-N
SMILESO=C=NCC1=CC=CC=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear, colourless to pale yellow
Formliquid
Refractive Index1.5230 - 1.5270 @20?C
Assay (GC)> 97.5%
Benzyl isocyanate, is used as a protected ammonia equivalent in the stereo selective ring-opening of chiral 2,3-epoxy alcohols (from Sharpless asymmetric epoxidation). The key step in the sequence involves intramolecular cyclization of the carbamate intermediate. It is also used in a multicomponent, stereospecific synthesis of 1,3-oxazinane-2,4-diones employing an Al-salph complex.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Benzyl isocyanate, is used as a protected ammonia equivalent in the stereo selective ring-opening of chiral 2,3-epoxy alcohols (from Sharpless asymmetric epoxidation). The key step in the sequence involves intramolecular cyclization of the carbamate intermediate. It is also used in a multicomponent, stereospecific synthesis of 1,3-oxazinane-2,4-diones employing an Al-salph complex.
Solubility
It hydrolyzes with water.
Notes
Moisture & heat Sensitive. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Keep away from alcohols, amines.
Benzyl isocyanate, is used as a protected ammonia equivalent in the stereo selective ring-opening of chiral 2,3-epoxy alcohols (from Sharpless asymmetric epoxidation). The key step in the sequence involves intramolecular cyclization of the carbamate intermediate. It is also used in a multicomponent, stereospecific synthesis of 1,3-oxazinane-2,4-diones employing an Al-salph complex.
Solubility
It hydrolyzes with water.
Notes
Moisture & heat Sensitive. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Keep away from alcohols, amines.
RUO – Research Use Only
General References:
- LL Ferstandig.; RA Scherrer. Mechanism of Isocyanate Reactions with Ethanol1. J. Am. Chem. Soc. 195981(18) , 4838-4842.
- DG Hoare.; DE Koshland Jr. A procedure for the selective modification of carboxyl groups in proteins. J. Am. Chem. Soc. 196688(9) , 2057-2058.
- Protected ammonia equivalent in the stereoselective ring-opening of chiral 2,3-epoxy alcohols (from Sharpless asymmetric epoxidation). The key step in the sequence involves intramolecular cyclization of the carbamate intermediate: J. Org. Chem., 50, 3752 (1985):
- For general reactions of isocyanates, see Appendix 3.