Search Thermo Fisher Scientific
Thermo Scientific Chemicals
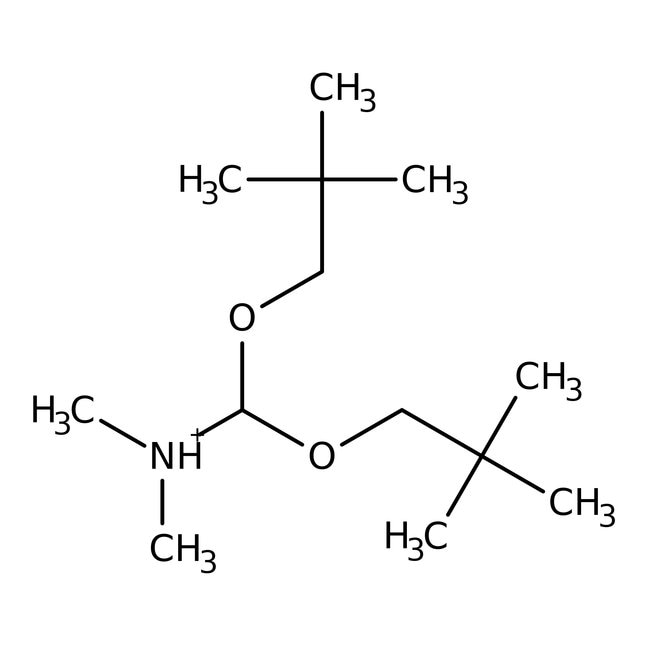
N,N-Dimethylformamide dineopentyl acetal, 98%, Thermo Scientific Chemicals
Catalog number L02728.18
also known as L02728-18
Price (USD)/ Each
301.00
-
Quantity:
50 g
Price (USD)/ Each
301.00
N,N-Dimethylformamide dineopentyl acetal, 98%, Thermo Scientific Chemicals
Catalog numberL02728.18
Price (USD)/ Each
301.00
-
Chemical Identifiers
CAS4909-78-8
Specifications Specification Sheet
Specification Sheet
Assay (Non-aqueous acid-base Titration)≥97.5 to ≤102.5%
Appearance (Color)Clear colorless
FormLiquid
Refractive Index1.4100-1.4140 @ 20?C
N,N-Dimethylformamide dineopentyl acetal helps in the lactonization of ω-hydroxyacids, giving lactones of up to 16-membered rings. It can be used to convert primary alcohols to alkylating agents for use in the alkylation of thiols. It is used in the esterification of Nα-9-fluorenylmethyloxycarbonylamino acids. It was used in the synthesis of 1,3-dialkyl, benzyl and cyclohexyl barbiturate derivatives. It was used as reagent during the synthesis of L-serine and L-cystine stereospecifically labeled with deuterium at the β-position.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
N,N-Dimethylformamide dineopentyl acetal helps in the lactonization of ω-hydroxyacids, giving lactones of up to 16-membered rings. It can be used to convert primary alcohols to alkylating agents for use in the alkylation of thiols. It is used in the esterification of Nα-9-fluorenylmethyloxycarbonylamino acids. It was used in the synthesis of 1,3-dialkyl, benzyl and cyclohexyl barbiturate derivatives. It was used as reagent during the synthesis of L-serine and L-cystine stereospecifically labeled with deuterium at the β-position.
Solubility
Hydrolyzes with water.
Notes
Moisture Sensitive. Incompatible with water and oxidizing agents. Store under dry inert gas. Protect from humidity and water.
N,N-Dimethylformamide dineopentyl acetal helps in the lactonization of ω-hydroxyacids, giving lactones of up to 16-membered rings. It can be used to convert primary alcohols to alkylating agents for use in the alkylation of thiols. It is used in the esterification of Nα-9-fluorenylmethyloxycarbonylamino acids. It was used in the synthesis of 1,3-dialkyl, benzyl and cyclohexyl barbiturate derivatives. It was used as reagent during the synthesis of L-serine and L-cystine stereospecifically labeled with deuterium at the β-position.
Solubility
Hydrolyzes with water.
Notes
Moisture Sensitive. Incompatible with water and oxidizing agents. Store under dry inert gas. Protect from humidity and water.
RUO – Research Use Only
General References:
- F Albericio; G Barany. Improved approach for anchoring N alpha-9-fluorenylmethyloxycarbonylamino acids as p-alkoxybenzyl esters in solid-phase peptide synthesis. International Journal of Peptide and Protein Reseach.1985, 26, (1), 92-97.
- F Albericio; G Barany. Application of N,N-dimethylformamide dineopentyl acetal for efficient anchoring of N alpha-9-fluorenylmethyloxycarbonylamino acids as p-alkoxybenzyl esters in solid-phase peptide synthesis. International Journal of Peptide and Protein Reseach.1985, 23, (4), 342-349.
- This hindered acetal, in conjunction with an alcohol, converts carboxylic acids to esters of that alcohol: Angew. Chem. Int. Ed., 3, 62 (1964); Helv. Chim. Acta, 48, 1746 (1965). For application to the lactonization of ω-hydroxyacids, giving lactones of up to 16-membered rings, in modest yield, see: Angew. Chem. Int. Ed., 16, 876 (1977). Similarly, can be used to convert primary alcohols to alkylating agents for use in the alkylation of thiols (e.g. 2-Mercaptopyrimidine, A13382): Tetrahedron Lett., 585 (1972).