Search Thermo Fisher Scientific
Thermo Scientific Chemicals
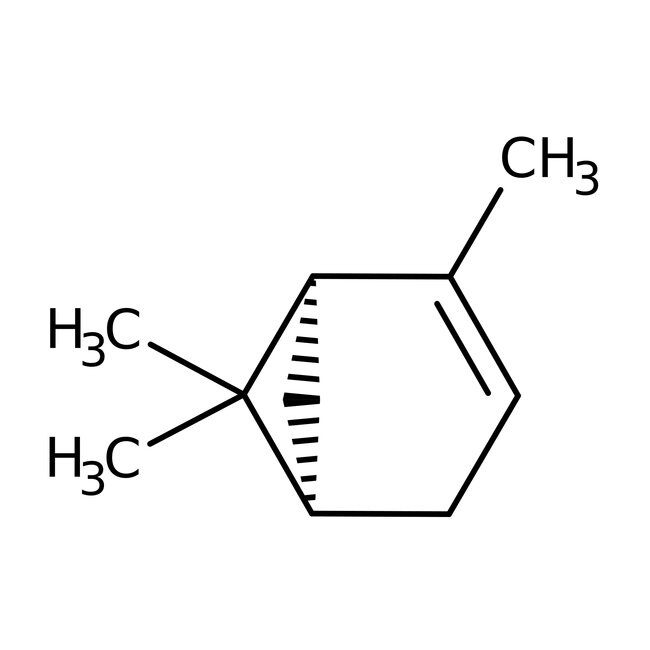
(+)-alpha-Pinene, 98%, Thermo Scientific Chemicals
Catalog number L04941.18
also known as L04941-18
Price (USD)/ Each
39.65
Online exclusive
44.40 Save 4.75 (11%)
-
Quantity:
50 g
Price (USD)/ Each
39.65
Online exclusive
44.40 Save 4.75 (11%)
(+)-alpha-Pinene, 98%, Thermo Scientific Chemicals
Catalog numberL04941.18
Price (USD)/ Each
39.65
Online exclusive
44.40 Save 4.75 (11%)
-
Chemical Identifiers
CAS7785-70-8
IUPAC Name(1R,5R)-2,6,6-trimethylbicyclo[3.1.1]hept-2-ene
Molecular FormulaC10H16
InChI KeyGRWFGVWFFZKLTI-RKDXNWHRSA-N
SMILESCC1=CC[C@@H]2C[C@H]1C2(C)C
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to yellow
FormLiquid
Assay (GC)≥97.5%
Optical Rotation40.0? to 48.0? (neat)
Refractive Index1.4635-1.4680 @ 20?C
(+)-alpha-Pinene is employed in the preparation of chiral hydroboration reagents. It is a bronchodilator in humans. α-Pinene is an anti-inflammatory and used as a broad-spectrum antibiotic.It exhibits activity as an acetylcholinesterase inhibitor, aiding memory. It is a biosynthetic base for CB2 ligands, such as HU-308.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
(+)-alpha-Pinene is employed in the preparation of chiral hydroboration reagents. It is a bronchodilator in humans. α-Pinene is an anti-inflammatory and used as a broad-spectrum antibiotic.It exhibits activity as an acetylcholinesterase inhibitor, aiding memory. It is a biosynthetic base for CB2 ligands, such as HU-308.
Solubility
Soluble in Ether, Alcohols, Chloroform. Not miscible in water.
Notes
Incompatible materials are Strong oxidizing agents. Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
(+)-alpha-Pinene is employed in the preparation of chiral hydroboration reagents. It is a bronchodilator in humans. α-Pinene is an anti-inflammatory and used as a broad-spectrum antibiotic.It exhibits activity as an acetylcholinesterase inhibitor, aiding memory. It is a biosynthetic base for CB2 ligands, such as HU-308.
Solubility
Soluble in Ether, Alcohols, Chloroform. Not miscible in water.
Notes
Incompatible materials are Strong oxidizing agents. Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
RUO – Research Use Only
General References:
- G. B. Pitman. trans-Verbenol and Alpha-Pinene: Their Utility in Manipulation of the Mountain Pine Beetle.Journal of Economic Entomology.1971, 64 426 - 430.
- Herbert C. Brown; Navalkishore N. Joshi. Hydroboration of terpenes. 9. A simple improved procedure for upgrading the optical purity of commercially available .alpha.- and .beta.-pinenes. Conversion of (+)-.alpha.-pinene to (+)-.beta.-pinene via hydroboration-isomerization.J. Org. Chem.1988, 53 (17), 4059-4062.
- Forms a chiral adduct with diborane, useful for asymmetric hydroborations with high optical yields: J. Am. Chem. Soc., 99, 5514 (1977). Recrystallization of the borane adduct can be used to obtain a pure single diastereomer: J. Org. Chem., 49, 945 (1984). For reviews of the synthesis of pure enantiomers via chiral organoboranes, see: Acc. Chem. Res., 21, 287 (1988); and of asymmetric reduction with chiral organoboranes based on ɑ-pinene: Acc. Chem. Res., 25, 16 (1992).