Search Thermo Fisher Scientific
Thermo Scientific Chemicals
2-Phenoxyaniline, 98%, Thermo Scientific Chemicals
Catalog number L05558.22
also known as L05558-22
Price (USD)/ Each
109.00
-
Quantity:
100 g
Price (USD)/ Each
109.00
2-Phenoxyaniline, 98%, Thermo Scientific Chemicals
Catalog numberL05558.22
Price (USD)/ Each
109.00
-
Chemical Identifiers
CAS2688-84-8
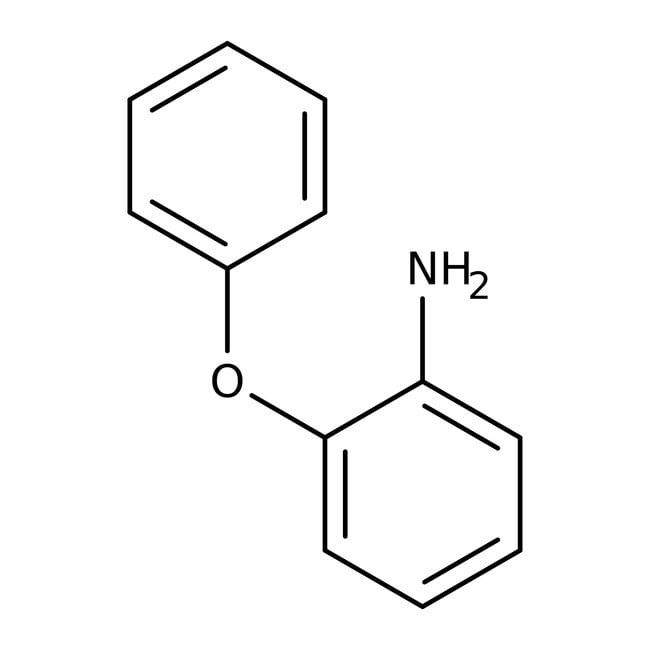
IUPAC Name2-phenoxyaniline
Molecular FormulaC12H11NO
InChI KeyNMFFUUFPJJOWHK-UHFFFAOYSA-N
SMILESNC1=CC=CC=C1OC1=CC=CC=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to cream to brown to dark brown
FormCrystals or powder or crystalline powder or lumps or clear liquid as melt
Assay (GC)≥97.5%
Identification (FTIR)Conforms
Melting Point (clear melt)41.0-50.0°C
2-Phenoxyaniline serves as an antiinflammatory agent that inhibits preferentially COX-2 over COX-1. It is used in the preparation of sodium primary amide complex, 4-methyl-2-(2-phenoxyphenyl)azo-phenol and 2-acetoaminodiphenyl ether. Further, it is used to form complexes with beta-cyclodextrin.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Phenoxyaniline serves as an antiinflammatory agent that inhibits preferentially COX-2 over COX-1. It is used in the preparation of sodium primary amide complex, 4-methyl-2-(2-phenoxyphenyl)azo-phenol and 2-acetoaminodiphenyl ether. Further, it is used to form complexes with beta-cyclodextrin.
Solubility
Slightly soluble in chloroform and methanol.
Notes
Incompatible with strong oxidizing agents.
2-Phenoxyaniline serves as an antiinflammatory agent that inhibits preferentially COX-2 over COX-1. It is used in the preparation of sodium primary amide complex, 4-methyl-2-(2-phenoxyphenyl)azo-phenol and 2-acetoaminodiphenyl ether. Further, it is used to form complexes with beta-cyclodextrin.
Solubility
Slightly soluble in chloroform and methanol.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Pinheiro, A. C.; Virgili, A. H.; Roisnel, T.; Kirillov, E.; Carpentier, J. F.; Casagrande, O. L. Ni(II) complexes bearing pyrrolide-imine ligands with pendant N-, O- and S-donor groups: synthesis, structural characterization and use in ethylene oligomerization. RSC Adv. 2015, 5 (111), 91524-91531.
- Li, M.; Chen, M.; Chen, C. Ring-opening polymerization of rac-lactide using anilinotropone-based aluminum complexes-sidearm effect on the catalysis. Polymer 2015, 64, 234-239.