Search Thermo Fisher Scientific
Thermo Scientific Chemicals
Cumyl hydroperoxide, tech. 80%, Thermo Scientific Chemicals
Catalog number: L06866.22
100 g, Each


Thermo Scientific Chemicals
Cumyl hydroperoxide, tech. 80%, Thermo Scientific Chemicals
Catalog number: L06866.22
100 g, Each
Quantity
Catalog number: L06866.22
also known as L06866-22
Price (USD)
36.60
Each
Quantity
-
Have Questions?
Chemical Identifiers
CAS
80-15-9
IUPAC Name
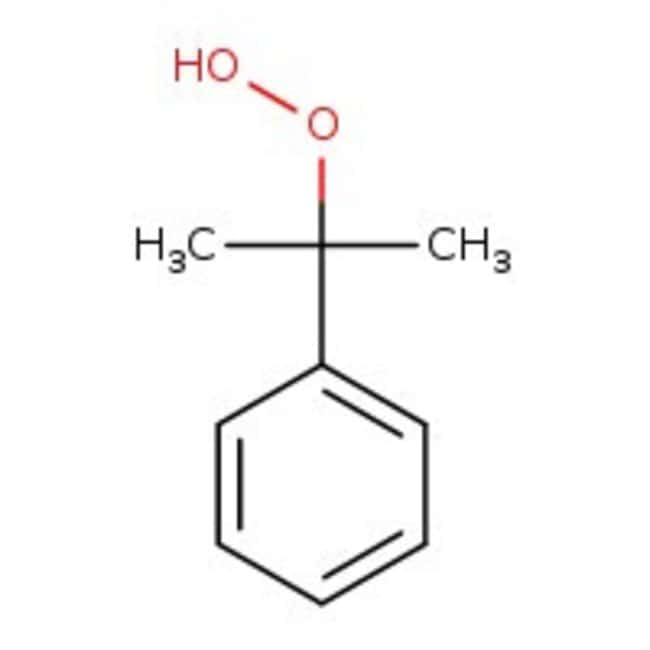
2-phenylpropane-2-peroxol
Molecular Formula
C9H12O2
InChI Key
YQHLDYVWEZKEOX-UHFFFAOYSA-N
SMILES
CC(C)(OO)C1=CC=CC=C1
Specifications
Identification (FTIR)
Conforms (U.K. specification)
Form
Liquid
Comment
Purchased in the U.K. and in other countries
Refractive Index
1.5170-1.5250 @ 20°C
Appearance (Color)
Clear colorless to pale yellow
Description
Cumene hydroperoxide is used in the preparation of polystyrene nanocapsules. It acts as a curing agent for polyester resins and as an oxidizer in organic chemical reactions. It serves as an initiator for radical polymerization especially for acrylate and methacrylate monomers. It also employed as an intermediate in the cumene process for developing phenol and acetone from benzene and propene. Further, it is used as an epoxidation reagent for allylic alcohols and fatty acid esters. In addition to this, it is also used to prepare methylstyrene, acetophenone and cumyl alcohol.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Cumene hydroperoxide is used in the preparation of polystyrene nanocapsules. It acts as a curing agent for polyester resins and as an oxidizer in organic chemical reactions. It serves as an initiator for radical polymerization especially for acrylate and methacrylate monomers. It also employed as an intermediate in the cumene process for developing phenol and acetone from benzene and propene. Further, it is used as an epoxidation reagent for allylic alcohols and fatty acid esters. In addition to this, it is also used to prepare methylstyrene, acetophenone and cumyl alcohol.
Solubility
Miscible with alcohol, acetone, ether, esters, hydrocarbons and chlorinated hydrocarbons. Slightly miscible with water.
Notes
Store in a cool place. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with powdered metals, organic materials, heavy metal salts, metal salts, combustible materials, acids, alkalis, reducing agents, rust, charcoal, amines, copper, lead, cobalt and cobalt oxides.
Cumene hydroperoxide is used in the preparation of polystyrene nanocapsules. It acts as a curing agent for polyester resins and as an oxidizer in organic chemical reactions. It serves as an initiator for radical polymerization especially for acrylate and methacrylate monomers. It also employed as an intermediate in the cumene process for developing phenol and acetone from benzene and propene. Further, it is used as an epoxidation reagent for allylic alcohols and fatty acid esters. In addition to this, it is also used to prepare methylstyrene, acetophenone and cumyl alcohol.
Solubility
Miscible with alcohol, acetone, ether, esters, hydrocarbons and chlorinated hydrocarbons. Slightly miscible with water.
Notes
Store in a cool place. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with powdered metals, organic materials, heavy metal salts, metal salts, combustible materials, acids, alkalis, reducing agents, rust, charcoal, amines, copper, lead, cobalt and cobalt oxides.
WARNING: Cancer – www.P65Warnings.ca.gov
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text