Search Thermo Fisher Scientific
Thermo Scientific Chemicals
2-Methyl-3-butyn-2-ol, 98%, Thermo Scientific Chemicals
Chemical Identifiers
CAS115-19-5
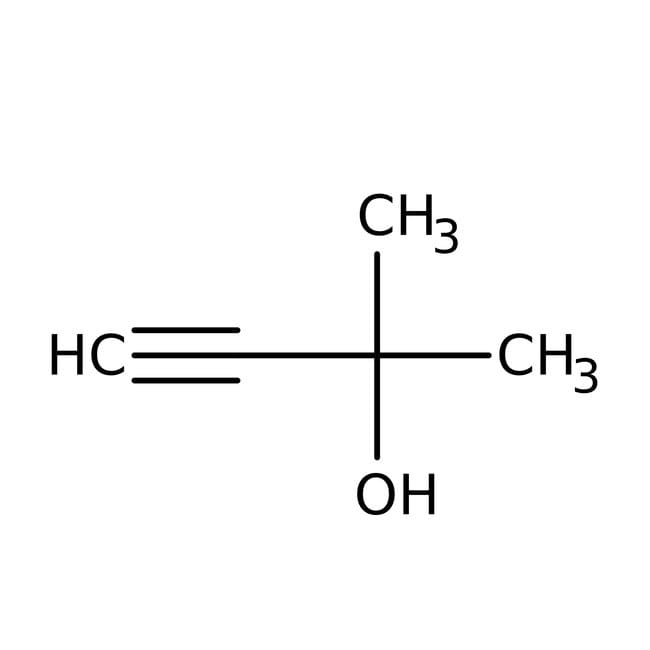
IUPAC Name2-methylbut-3-yn-2-ol
Molecular FormulaC5H8O
InChI KeyCEBKHWWANWSNTI-UHFFFAOYSA-N
SMILESCC(C)(O)C#C
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear, colourless
Assay (GC)>97.5%
Refractive Index1.4190 - 1.4230 @20?C
Formliquid
2-Methyl-3-butyn-2-ol is used as an intermediate in the manufacture of products for the agrochemical and specialty chemical industry.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Methyl-3-butyn-2-ol is used as an intermediate in the manufacture of products for the agrochemical and specialty chemical industry.
Solubility
Fully miscible.
Notes
Keep container tightly closed. Store away from oxidizing agents.
2-Methyl-3-butyn-2-ol is used as an intermediate in the manufacture of products for the agrochemical and specialty chemical industry.
Solubility
Fully miscible.
Notes
Keep container tightly closed. Store away from oxidizing agents.
RUO – Research Use Only
General References:
- Edward T. Sabourin.; Anatoli Onopchenko. A convenient synthesis of 4-ethynylphthalic anhydride via 2-methyl-3-butyn-2-ol. J. Org. Chem. 1983, 48, (25), 5135-5137.
- D. Boyall.; F. López.; H. Sasaki.; D. Frantz.; E. M. Carreira. Enantioselective Addition of 2-Methyl-3-butyn-2-ol to Aldehydes: Preparation of 3-Hydroxy-1-butynes. Org. Lett. 2000, 2, (26), 4233-4236.
- Adduct of acetylene and acetone which acts as an acetylene equivalent in the Pd-catalyzed (Sonogashira) coupling reaction with aryl or heteroaryl halides, permitting a one-pot diarylation by different halides: Synthesis, 571 (1984):
- Alternatively, the protecting group can be removed from the intermediate by base to give monoarylacetylenes: J. Org. Chem., 48, 5135 (1983); 57, 6998 (1992). For an improved procedure, see: Synthesis, 589 (1996).