Search Thermo Fisher Scientific
Thermo Scientific Chemicals
Triethyl methanetricarboxylate, 98%, Thermo Scientific Chemicals
Catalog number L08356.14
also known as L08356-14
Price (USD)/ Each
82.60
-
Quantity:
25 g
Price (USD)/ Each
82.60
Triethyl methanetricarboxylate, 98%, Thermo Scientific Chemicals
Catalog numberL08356.14
Price (USD)/ Each
82.60
-
Chemical Identifiers
CAS6279-86-3
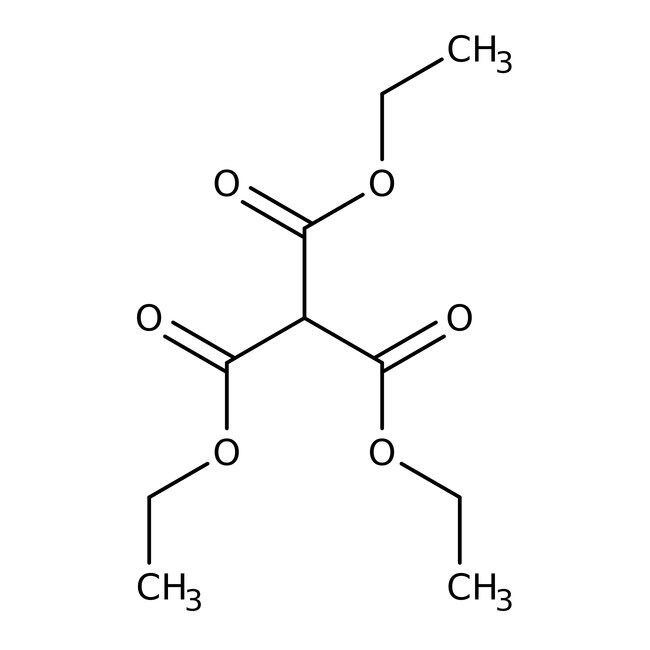
IUPAC Nametriethyl methanetricarboxylate
Molecular FormulaC10H16O6
InChI KeyAGZPNUZBDCYTBB-UHFFFAOYSA-N
SMILESCCOC(=O)C(C(=O)OCC)C(=O)OCC
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Colorless to white to pale yellow
FormCrystals or powder or crystalline powder or fused solid or clear liquid or viscous liquid as melt
Identification (FTIR)Conforms
Refractive Index1.4235-1.4275 @ 20?C
Assay (GC)≥97.5%
Methanetricarboxylates have been used as blocked malonic esters, restricting reaction with alkyl halides to monoalkylation. The unwanted carboxyl group can be removed by treatment with Na alkoxide, LDA or BCl3. Provides a useful 2-carbon chain extension method that adds to Michael acceptors under phase-transfer conditions. Triethyl Methanetricarboxylate is used in the synthesis of novel inhibitors of Hsp90. It is also used in the preparation of novel dihydroquinoline-3-carboxylic acids that function as HIV-1 integrase inhibitors.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Methanetricarboxylates have been used as “blocked” malonic esters, restricting reaction with alkyl halides to monoalkylation. The unwanted carboxyl group can be removed by treatment with Na alkoxide, LDA or BCl3. Provides a useful 2-carbon chain extension method that adds to Michael acceptors under phase-transfer conditions. Triethyl Methanetricarboxylate is used in the synthesis of novel inhibitors of Hsp90. It is also used in the preparation of novel dihydroquinoline-3-carboxylic acids that function as HIV-1 integrase inhibitors.
Solubility
Insoluble in water.
Notes
Store away from oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
Methanetricarboxylates have been used as “blocked” malonic esters, restricting reaction with alkyl halides to monoalkylation. The unwanted carboxyl group can be removed by treatment with Na alkoxide, LDA or BCl3. Provides a useful 2-carbon chain extension method that adds to Michael acceptors under phase-transfer conditions. Triethyl Methanetricarboxylate is used in the synthesis of novel inhibitors of Hsp90. It is also used in the preparation of novel dihydroquinoline-3-carboxylic acids that function as HIV-1 integrase inhibitors.
Solubility
Insoluble in water.
Notes
Store away from oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
RUO – Research Use Only
General References:
- Giancarlo Cravotto, et al. Dehydrative alkylation of alcohols with triethyl methanetricarboxylate under Mitsunobu conditions.Tetrahedron.,1996,50(40), 13007-13016.
- Teruaki Mukaiyama, et al. Stereoselective Carbon-Carbon Bond Forming Reactions between Various Chiral Alkyl Aryl Carbinols and Triethyl Methanetricarboxylate by Oxidation-Reduction Condensation Using Alkyl Diphenylphosphinites.Chem. Lett.,2005,34(12), 1676-1677.
- Methanetricarboxylates have been used as blocked malonic esters, restricting reaction with alkyl halides to monoalkylation. The unwanted carboxyl group can be removed by treatment with Na alkoxide, LDA or BCl3: J. Org. Chem., 44, 3492 (1979). Adds to Michael acceptors under phase-transfer conditions, providing a useful 2-carbon chain extension method: Synthesis, 1125 (1990):
- Under similar conditions, diethyl malonate gives only 36% yield.
- The tricarboxylate also undergoes free-radical addition to the terminal position of alkenes: Angew. Chem. Int. Ed., 5, 586 (1966). Arylation with electron-rich aromatics is promoted by Mn(III): Synthesis, 567 (1991).