Search Thermo Fisher Scientific
Thermo Scientific Chemicals
Thiazole, 99%, Thermo Scientific Chemicals
Catalog number L09970.03
also known as L09970-03
Price (USD)/ Each
40.30
-
Quantity:
1 g
Price (USD)/ Each
40.30
Thiazole, 99%, Thermo Scientific Chemicals
Catalog numberL09970.03
Price (USD)/ Each
40.30
-
Chemical Identifiers
CAS288-47-1
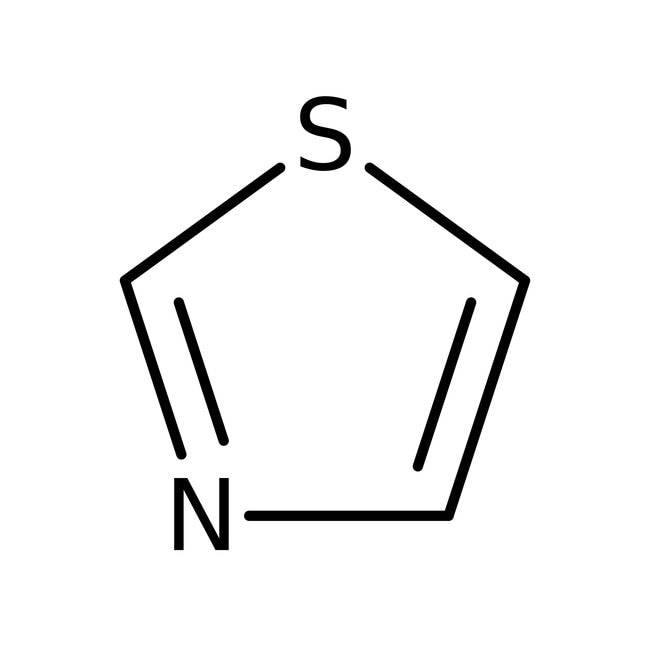
IUPAC Name1,3-thiazole
Molecular FormulaC3H3NS
InChI KeyFZWLAAWBMGSTSO-UHFFFAOYSA-N
SMILESS1C=CN=C1
View more
Specifications Specification Sheet
Specification Sheet
FormLiquid
Refractive Index1.5330-1.5395 @ 20°C
Appearance (Color)Clear colorless to yellow
Assay (GC)≥97.5%
Thiazole is used as a flavoring agent and in the preparation of dyes and rubber accelerators. It serves as a component of the vitamin thiamine (B1). It acts as a protected formyl group used in natural product synthesis. It reacts with alkyl lithium and Grignard’s reagent to prepare organometallic complexes. It is involved in the electrophilic aromatic substitution and nucleophilic aromatic substitution at C-5 and C-2 positions respectively. Further, it undergoes alkylation reaction to get thiazolium cation, which is used as a catalyst in the Stetter reaction and the Benzoin condensation. In addition to this, it is involved in the preparation of alagebrium.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Thiazole is used as a flavoring agent and in the preparation of dyes and rubber accelerators. It serves as a component of the vitamin thiamine (B1). It acts as a protected formyl group used in natural product synthesis. It reacts with alkyl lithium and Grignard’s reagent to prepare organometallic complexes. It is involved in the electrophilic aromatic substitution and nucleophilic aromatic substitution at C-5 and C-2 positions respectively. Further, it undergoes alkylation reaction to get thiazolium cation, which is used as a catalyst in the Stetter reaction and the Benzoin condensation. In addition to this, it is involved in the preparation of alagebrium.
Solubility
Soluble in ethanol and ether. Slightly soluble in water.
Notes
Air and light sensitive. Incompatible with acids, acid chlorides, acid anhydrides, strong oxidizing agents and carbon dioxide.
Thiazole is used as a flavoring agent and in the preparation of dyes and rubber accelerators. It serves as a component of the vitamin thiamine (B1). It acts as a protected formyl group used in natural product synthesis. It reacts with alkyl lithium and Grignard’s reagent to prepare organometallic complexes. It is involved in the electrophilic aromatic substitution and nucleophilic aromatic substitution at C-5 and C-2 positions respectively. Further, it undergoes alkylation reaction to get thiazolium cation, which is used as a catalyst in the Stetter reaction and the Benzoin condensation. In addition to this, it is involved in the preparation of alagebrium.
Solubility
Soluble in ethanol and ether. Slightly soluble in water.
Notes
Air and light sensitive. Incompatible with acids, acid chlorides, acid anhydrides, strong oxidizing agents and carbon dioxide.
RUO – Research Use Only
General References:
- For an example of 2-lithiation, see: J. Med. Chem., 37, 3492 (1994). For other uses of 2-lithiothiazole, see 2-Bromothiazole, A14838.
- Li, F.; Meng, F.; Wang, Y.; Zhu, C.; Cheng, Y. Polymer-based fluorescence sensor incorporating thiazole moiety for direct and visual detection of Hg2+ and Ag+. Tetrahedron 2015, 71 (11), 1700-1704.
- Mallia, C. J.; Englert, L.; Walter, G. C.; Baxendale, I. R. Thiazole formation through a modified Gewald reaction. Beilstein J. Org. Chem. 2015, 11, 875-883.