Search Thermo Fisher Scientific
Thermo Scientific Chemicals
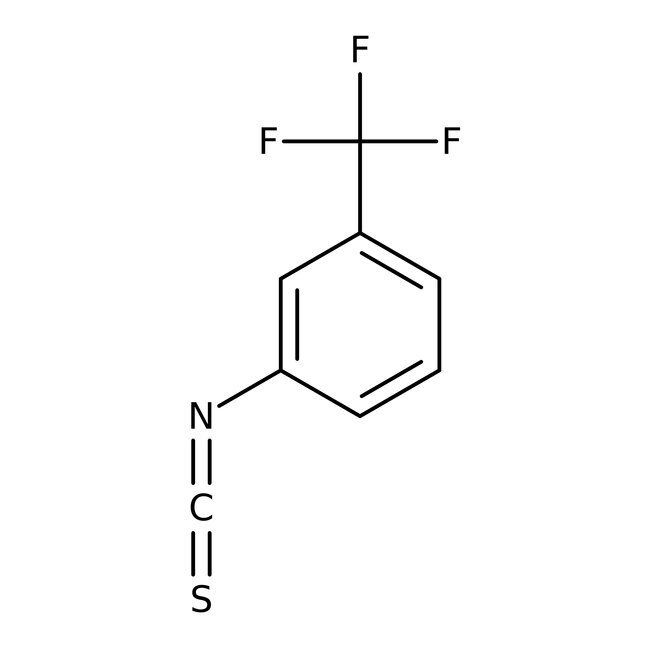
3-(Trifluoromethyl)phenyl isothiocyanate, 98%, Thermo Scientific Chemicals
Catalog number L11646.14
also known as L11646-14
Price (USD)/ Each
185.00
-
Quantity:
25 g
Price (USD)/ Each
185.00
3-(Trifluoromethyl)phenyl isothiocyanate, 98%, Thermo Scientific Chemicals
Catalog numberL11646.14
Price (USD)/ Each
185.00
-
Chemical Identifiers
CAS1840-19-3
IUPAC Name1-isothiocyanato-3-(trifluoromethyl)benzene
Molecular FormulaC8H4F3NS
InChI KeyGFEPANUKFYVALF-UHFFFAOYSA-N
SMILESFC(F)(F)C1=CC=CC(=C1)N=C=S
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to yellow
FormLiquid
Assay (GC)≥97.5%
CommentSpecification differs for U.S. and non-U.S. material where indicated
Identification (FTIR)Conforms (non-U.S. specification)
View more
Used as pharamaceutical intermediate.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Used as pharamaceutical intermediate.
Solubility
Hydrolyzes in water.
Notes
Keep container tightly sealed. Store in cool, dry conditions in well sealed containers. Incompatible with oxidizing agents. It is sensitive to moisture.
Used as pharamaceutical intermediate.
Solubility
Hydrolyzes in water.
Notes
Keep container tightly sealed. Store in cool, dry conditions in well sealed containers. Incompatible with oxidizing agents. It is sensitive to moisture.
RUO – Research Use Only
General References:
- Ha-Young Kim.; Se Hun Kwak.; Gee-Hyung Lee.; Young-Dae Gong. Copper-catalyzed synthesis of 3-substituted-5-amino-1,2,4-thiadiazoles via intramolecular N-S bond formation. Tetrahedron. 2014, 70 (45), 8737-8743.
- Sara Van Poecke.; Hélène Munier-Lehmann.; Olivier Helynck.; Matheus Froeyend.; Serge Van Calenbergh. Synthesis and inhibitory activity of thymidine analogues targeting Mycobacterium tuberculosis thymidine monophosphate kinase. Bioorganic & Medicinal Chemistry. 2011, 19 (24), 7603-7611.