Search Thermo Fisher Scientific
Thermo Scientific Chemicals
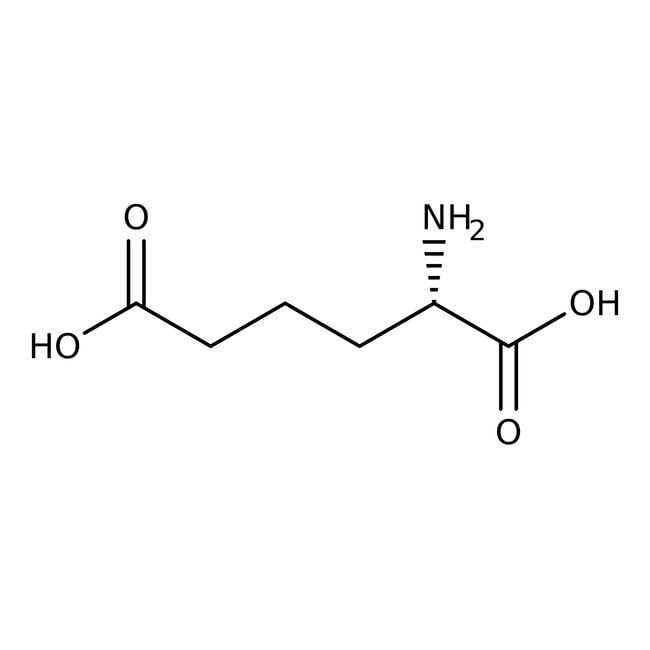
L-2-Aminoadipic acid, 98%, Thermo Scientific Chemicals
Catalog number L13924.03
also known as L13924-03
Price (USD)/ Each
84.65
Online exclusive
93.80 Save 9.15 (10%)
-
Quantity:
1 g
Price (USD)/ Each
84.65
Online exclusive
93.80 Save 9.15 (10%)
L-2-Aminoadipic acid, 98%, Thermo Scientific Chemicals
Catalog numberL13924.03
Price (USD)/ Each
84.65
Online exclusive
93.80 Save 9.15 (10%)
-
Chemical Identifiers
CAS1118-90-7
IUPAC Name(2S)-2-aminohexanedioic acid
Molecular FormulaC6H11NO4
InChI KeyOYIFNHCXNCRBQI-BYPYZUCNSA-N
SMILESN[C@@H](CCCC(O)=O)C(O)=O
View more
Specifications Specification Sheet
Specification Sheet
Optical Rotation+24° ± 2° (c=2 in 5N HCl, non-U.S. specification)
Appearance (Color)White
FormPowder
Acid-base back titration≥97.5 to ≤102.5% (non-U.S. specification)
Assay (Silylated GC)≥97.5% (non-U.S. specification)
View more
L-2-Aminoadipic acid is an important raw material and intermediate used in organic synthesis, pharmaceuticals and agrochemicals.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
L-2-Aminoadipic acid is an important raw material and intermediate used in organic synthesis, pharmaceuticals and agrochemicals.
Solubility
Soluble in 1N HCl (50 mg/ml) and water (slightly).
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with oxidizing agents.
L-2-Aminoadipic acid is an important raw material and intermediate used in organic synthesis, pharmaceuticals and agrochemicals.
Solubility
Soluble in 1N HCl (50 mg/ml) and water (slightly).
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Tamas Szirtes.; Lajos Kisfaludy.; Eva Palosi.; Laszlo Szporny. Synthesis of thyrotropin-releasing hormone analogs. 2. Tripeptides structurally greatly different from TRH with high central nervous system activity. J. Am. Chem. Soc. 1986, 29 (9), 1654-1658.
- Gianfranco Pasut.; Fabiana Canal.; Lisa Dalla Via.; Silvia Arpicco.; Francesco M. Veronese.; Oddone Schiavon. Antitumoral activity of PEG-gemcitabine prodrugs targeted by folic acid. Journal of Controlled Release. 2008, 127 (3), 239-248.