Search Thermo Fisher Scientific
Thermo Scientific Chemicals
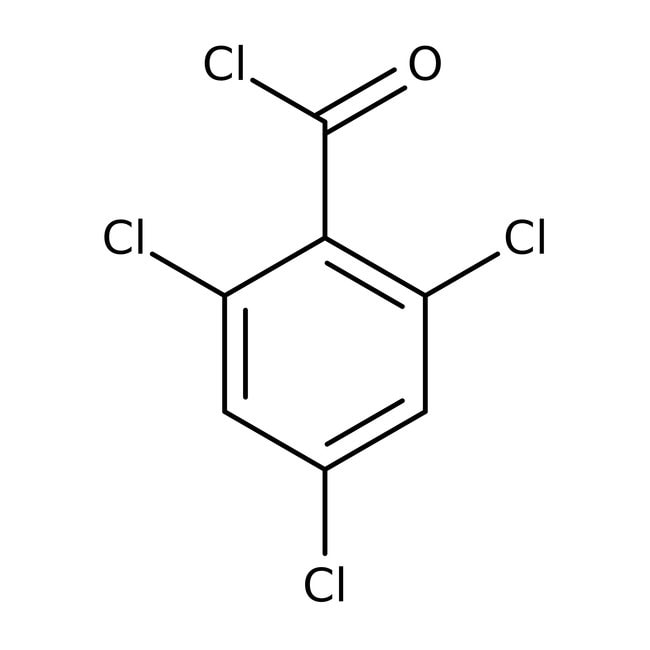
2,4,6-Trichlorobenzoyl chloride, 98%, Thermo Scientific Chemicals
Catalog number L14159.14
also known as L14159-14
Price (USD)/ Each
217.65
Online exclusive
242.00 Save 24.35 (10%)
-
Quantity:
25 g
Price (USD)/ Each
217.65
Online exclusive
242.00 Save 24.35 (10%)
2,4,6-Trichlorobenzoyl chloride, 98%, Thermo Scientific Chemicals
Catalog numberL14159.14
Price (USD)/ Each
217.65
Online exclusive
242.00 Save 24.35 (10%)
-
Chemical Identifiers
CAS4136-95-2
IUPAC Name2,4,6-trichlorobenzoyl chloride
Molecular FormulaC7H2Cl4O
InChI KeyOZGSEIVTQLXWRO-UHFFFAOYSA-N
SMILESClC(=O)C1=C(Cl)C=C(Cl)C=C1Cl
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to yellow
FormLiquid or viscous liquid
Assay (Titration ex Chloride)≥97.5 to ≤102.5%
Assay (GC)≥97.5%
Refractive Index1.5740-1.5780 @ 20?C
2,4,6-Trichlorobenzoyl chloride is used in the preparation of γ-lactone and δ-lactone, aliphatic aromatic anhydrides, required for the synthesis of amphiphilic hyaluronan, mixed anhydride, required for the synthesis of angelate esters, synthesis of both spongistatin 1 and spongistatin 2 and large-ring lactones in high yields.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2,4,6-Trichlorobenzoyl chloride is used in the preparation of γ-lactone and δ-lactone, aliphatic aromatic anhydrides, required for the synthesis of amphiphilic hyaluronan, mixed anhydride, required for the synthesis of angelate esters, synthesis of both spongistatin 1 and spongistatin 2 and large-ring lactones in high yields.
Solubility
Reacts with water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are opened must be carefully resealed and kept upright to prevent leakage. It is sensitive to moisture. Incompatible with strong bases and strong oxidizing agents.
2,4,6-Trichlorobenzoyl chloride is used in the preparation of γ-lactone and δ-lactone, aliphatic aromatic anhydrides, required for the synthesis of amphiphilic hyaluronan, mixed anhydride, required for the synthesis of angelate esters, synthesis of both spongistatin 1 and spongistatin 2 and large-ring lactones in high yields.
Solubility
Reacts with water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are opened must be carefully resealed and kept upright to prevent leakage. It is sensitive to moisture. Incompatible with strong bases and strong oxidizing agents.
RUO – Research Use Only
General References:
- Gloria Huerta-Angeles.; Martin Bobek.; Eva Příkopová.; Daniela Šmejkalová.; Vladimír Velebný. Novel synthetic method for the preparation of amphiphilic hyaluronan by means of aliphatic aromatic anhydrides.Carbohydrate Polymers 2014, 111 883-891.
- Machiko Ono.; Keisuke Kato.; Hiroyuki Akita. Synthesis of γ- and δ-lactone natural products by employing a trans-cis isomerization/lactonization strategy. Chemical & Pharmaceutical Bulletin. 2013, 61 (4), 464-470.
- Reagent, introduced by Yamaguchi, which forms mixed anhydrides with acids, useful in the synthesis of esters: Bull. Chem. Soc. Jpn., 52, 1989 (1979), or lactones including macrolides: Chem. Lett., 1021 (1979). Superior to mesitylenesulfonyl chloride for macrolactonization, which has been achieved in the presence of DMAP without the need for high dilution: J. Org. Chem., 55, 7 (1990); Tetrahedron Lett., 31, 6367 (1990).