Search Thermo Fisher Scientific
Thermo Scientific Chemicals
Hexamethyldisilazane, Electronic grade, 99+%, Thermo Scientific Chemicals
Catalog number L16519.AE
also known as L16519-AE
Price (USD)/ Each
79.65
Online exclusive
88.00 Save 8.35 (9%)
-
Quantity:
100 mL
Price (USD)/ Each
79.65
Online exclusive
88.00 Save 8.35 (9%)
Hexamethyldisilazane, Electronic grade, 99+%, Thermo Scientific Chemicals
Catalog numberL16519.AE
Price (USD)/ Each
79.65
Online exclusive
88.00 Save 8.35 (9%)
-
Chemical Identifiers
CAS999-97-3
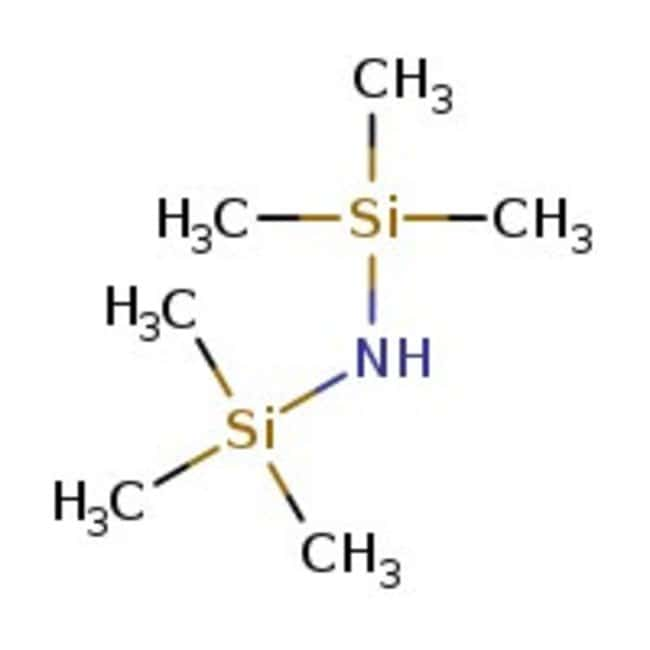
IUPAC Namebis(trimethylsilyl)amine
Molecular FormulaC6H19NSi2
InChI KeyFFUAGWLWBBFQJT-UHFFFAOYSA-N
SMILESC[Si](C)(C)N[Si](C)(C)C
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless
Identification (FTIR)Conforms
Total Metal Impurities≤1150ppm
FormLiquid
Colour (Hazen Units)≤10
View more
Hexamethyldisilazane is used as a solvent in organic synthesis and organometallic chemistry. It is often used as an adhesion promoter for photoresist in photolithography. Further, it is used for the preparation of trimethylsilyl ethers from hydroxy compounds. It is used as an alternative to critical point drying during sample preparation in electron microscopy. It is added to analyte to get silylated diagnostic products during pyrolysis in gas chromatography- mass spectrometry.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Hexamethyldisilazane is used as a solvent in organic synthesis and organometallic chemistry. It is often used as an adhesion promoter for photoresist in photolithography. Further, it is used for the preparation of trimethylsilyl ethers from hydroxy compounds. It is used as an alternative to critical point drying during sample preparation in electron microscopy. It is added to analyte to get silylated diagnostic products during pyrolysis in gas chromatography- mass spectrometry.
Solubility
Miscible with methanol, ethyl ether, chloroform and benzene. Immiscible with water.
Notes
Incompatible with strong oxidizing agents and strong acids.
Hexamethyldisilazane is used as a solvent in organic synthesis and organometallic chemistry. It is often used as an adhesion promoter for photoresist in photolithography. Further, it is used for the preparation of trimethylsilyl ethers from hydroxy compounds. It is used as an alternative to critical point drying during sample preparation in electron microscopy. It is added to analyte to get silylated diagnostic products during pyrolysis in gas chromatography- mass spectrometry.
Solubility
Miscible with methanol, ethyl ether, chloroform and benzene. Immiscible with water.
Notes
Incompatible with strong oxidizing agents and strong acids.
RUO – Research Use Only
General References:
- Convenient, mild silylating reagent which generates gaseous ammonia as the only by-product (see Appendix 4). Non-acidic substrates normally require a catalyst.
- Silylation of alcohols, including carbohydrates, is catalyzed by TMS chloride: J. Org. Chem., 23, 50 (1958); J. Am. Chem. Soc., 85, 2497 (1963); Chem. Ind. (London), 794 (1984). Silylation of phenols occurs readily, see also: Liebigs Ann. Chem., 20 (1973).
- HMDS in the presence of TMS chloride permits the selective O-silylation of amino alcohols: Synthesis, 990 (1988). Alcohols and phenols can be silylated in the presence of amines and thiols with ZnCl2 as catalyst: Synth. Commun., 23, 1633 (1993).
- For conversion of carbonyl groups to silyl enol ethers, see Iodotrimethyl silane, A12902.
- A range of catalysts for silylation with HMDS, including saccharin and sodium saccharin, has been recommended: J. Org. Chem., 47, 3966 (1982), for silylation of alcohols, phenols, thiols, carboxylic acids, amides, thioamides, hydroxamic acids, hydrazides, NH-groups of heterocycles, hydrazines, 1,3-diketones, etc. The use of TBAF (0.02 eq.) or iodine (0.01 eq.) also provide mild procedures for O-silylation: Tetrahedron Lett., 35, 8409 (1994); J. Org. Chem., 65, 7228 (2000).
- Can also function as a protected form of ammonia, e.g. to convert acid chlorides to primary amides: Synthesis, 517 (1985), and substituted maleic anhydrides to maleimides: Tetrahedron Lett., 31, 5201 (1990).
- In combination with DMSO, thiols are oxidized to disulfides under nearly neutral conditions: Synlett, 346 (2002).
- The Na, Li and K derivatives are useful strong bases; see Sodium bis(trimethyl silyl) amide, L13352, Lithium bis(trimethyl silyl) amide, L15012, and Potassium bis(trimethyl silyl) amide, L15022.