Search Thermo Fisher Scientific
Thermo Scientific Chemicals
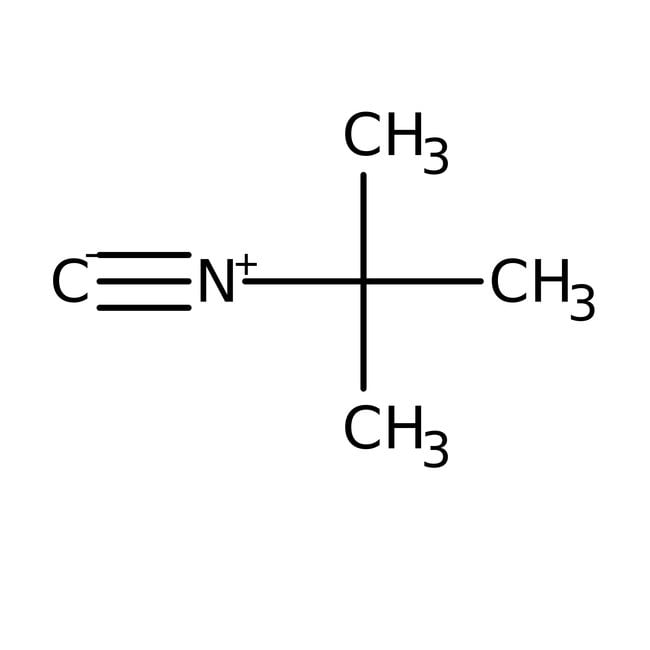
tert-Butyl isocyanide, 98%, Thermo Scientific Chemicals
Catalog number L19747.06
Price (USD)/ Each
132.65
Special Offer
Online exclusive
Ends: 31-Dec-2024
156.00 Save 23.35 (15%)
-
Quantity:
5 g
Price (USD)/ Each
132.65
Special Offer
Online exclusive
Ends: 31-Dec-2024
156.00 Save 23.35 (15%)
tert-Butyl isocyanide, 98%, Thermo Scientific Chemicals
Catalog numberL19747.06
Price (USD)/ Each
132.65
Special Offer
Online exclusive
Ends: 31-Dec-2024
156.00 Save 23.35 (15%)
-
Chemical Identifiers
CAS7188-38-7
IUPAC Name2-isocyano-2-methylpropane
Molecular FormulaC5H9N
InChI KeyFAGLEPBREOXSAC-UHFFFAOYSA-N
SMILESCC(C)(C)[N+]#[C-]
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to pale yellow
FormLiquid
Identification (FTIR)Conforms
Assay (GC)≥97.5%
Refractive Index1.3735-1.3775 @ 20?C
tert-Butyl isocyanide is used in a tin-free procedure for alkyl radical reactions in the presence of a free-radical initiator. It is a useful intermediate in multicomponent reactions. It is also used in the synthesis of coumarines, 4H-chromenes, isoxazolines and to trap 2-cyclopropylidene-1,3-diones.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
tert-Butyl isocyanide is used in a tin-free procedure for alkyl radical reactions in the presence of a free-radical initiator. It is a useful intermediate in multicomponent reactions. It is also used in the synthesis of coumarines, 4H-chromenes, isoxazolines and to trap 2-cyclopropylidene-1,3-diones.
Solubility
Soluble in organic solvents such as ethanol, methanol, ether, toluene and dichloromethane.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with oxidizing agents.
tert-Butyl isocyanide is used in a tin-free procedure for alkyl radical reactions in the presence of a free-radical initiator. It is a useful intermediate in multicomponent reactions. It is also used in the synthesis of coumarines, 4H-chromenes, isoxazolines and to trap 2-cyclopropylidene-1,3-diones.
Solubility
Soluble in organic solvents such as ethanol, methanol, ether, toluene and dichloromethane.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Bonggeun Shong.; Keith T Wong.; Stacey F Bent. Strong carbon-surface dative bond formation by tert-butyl isocyanide on the Ge(100)-2 x 1 surface. Journal of the American Chemical Society. 2014, 136 (16), 5848-5851.
- Jack E. Baldwin.; Stephen B. Haber, Carolyn Hoskins, Lawrence I. Kruse. Synthesis of .beta.,.gamma.-unsaturated amino acids. J. Org. Chem. 1977, 42 (7), 1239-1241.
- In the presence of a free-radical initiator, can be used in a tin-free procedure for alkyl radical reactions: Angew. Chem. Int. Ed., 43, 3598 (2004).
- Useful intermediate in multicomponent reactions. For example, forms a reactive 1 : 1 adduct with acetylenic esters, e.g. Dimethyl acetyl enedicarboxyl ate, A11437, which can be trapped with strong C-H acids, such as 4-Hydroxy-6-methyl-2-pyrone, L11457: