Search Thermo Fisher Scientific
Thermo Scientific Chemicals
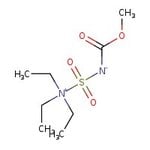
Burgess Reagent, 96%
Catalog number: L20155.03
1 g, Each


Thermo Scientific Chemicals
Burgess Reagent, 96%
Catalog number: L20155.03
1 g, Each
Quantity
Catalog number: L20155.03
also known as L20155-03
Price (USD)
86.00
Each
Quantity
-
Have Questions?
Chemical Identifiers
CAS
29684-56-8
IUPAC Name
(methoxycarbonyl)[(triethylazaniumyl)sulfonyl]azanide
Molecular Formula
C8H18N2O4S
InChI Key
YSHOWEKUVWPFNR-UHFFFAOYSA-N
SMILES
CC[N+](CC)(CC)S(=O)(=O)[N-]C(=O)OC
Specifications
Form
Crystals or powder or crystalline powder or lumps
Appearance (Color)
White to cream to yellow
Assay (HPLC)
≥95.0%
Identification (FTIR)
Conforms
Description
Burgess reagent is a selective dehydrating reagent used in organic synthesis. It is used to convert secondary and tertiary alcohol, with an adjacent proton, into alkenes. Burgess reagent is also utilized to promote great synthetic values in medicinal chemistry. It has been used in dehydration of primary amides, formamides and primary nitroalkanes to generate nitriles, isocyanides and nitrile oxides respectively. It forms urethanes when it reacts with primary alcohols.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Burgess reagent is a selective dehydrating reagent used in organic synthesis. It is used to convert secondary and tertiary alcohol, with an adjacent proton, into alkenes. Burgess reagent is also utilized to promote great synthetic values in medicinal chemistry. It has been used in dehydration of primary amides, formamides and primary nitroalkanes to generate nitriles, isocyanides and nitrile oxides respectively. It forms urethanes when it reacts with primary alcohols.
Solubility
Soluble in organic solvents.
Notes
Store in cool place. Moisture sensitive. Incompatible with strong oxidizing agents.
Burgess reagent is a selective dehydrating reagent used in organic synthesis. It is used to convert secondary and tertiary alcohol, with an adjacent proton, into alkenes. Burgess reagent is also utilized to promote great synthetic values in medicinal chemistry. It has been used in dehydration of primary amides, formamides and primary nitroalkanes to generate nitriles, isocyanides and nitrile oxides respectively. It forms urethanes when it reacts with primary alcohols.
Solubility
Soluble in organic solvents.
Notes
Store in cool place. Moisture sensitive. Incompatible with strong oxidizing agents.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text