Search Thermo Fisher Scientific
Thermo Scientific Chemicals
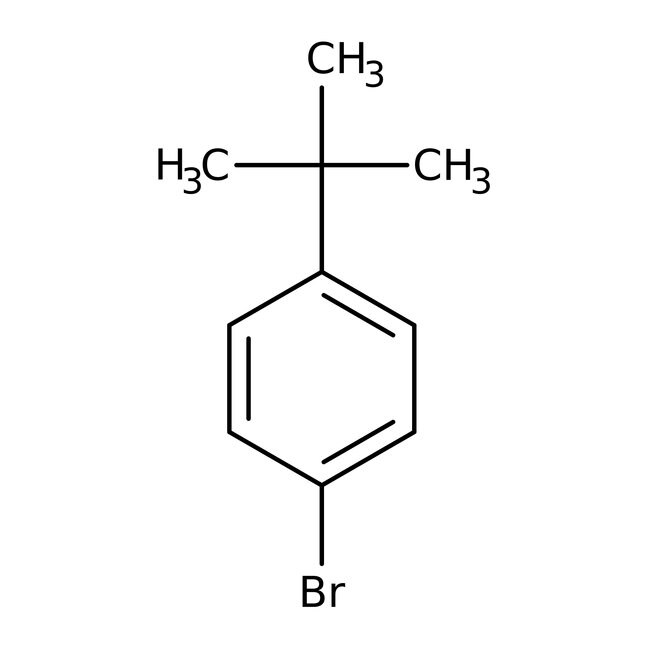
1-Bromo-4-tert-butylbenzene, 97%, Thermo Scientific Chemicals
Catalog number A15924.36
also known as A15924-36
Price (EUR)/ Each
706,66
Online exclusive
794.00 Save 87,34 (11%)
-
Quantity:
500 g
Price (EUR)/ Each
706,66
Online exclusive
794.00 Save 87,34 (11%)
1-Bromo-4-tert-butylbenzene, 97%, Thermo Scientific Chemicals
Catalog numberA15924.36
Price (EUR)/ Each
706,66
Online exclusive
794.00 Save 87,34 (11%)
-
Chemical Identifiers
CAS3972-65-4
IUPAC Name1-bromo-4-tert-butylbenzene
Molecular FormulaC10H13Br
InChI KeyXHCAGOVGSDHHNP-UHFFFAOYSA-N
SMILESCC(C)(C)C1=CC=C(Br)C=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to pale yellow
Assay (GC)≥96.0%
Refractive Index1.5310-1.5350 @ 20?C
Identification (FTIR)Conforms
FormLiquid
1-Bromo-4-tert-butylbenzene was used in the synthesis of 4-tert-butyl-phenylboronic acid1, 1-deoxy analogs of CP-47,497 (n = 0 to 7) and 1-deoxy analogs of CP-55,940 (n = 0 to 7). It undergoes lithium-bromide exchange reactions with n-butyllithium and tert-butyllithium at 0°C in various solvents.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-Bromo-4-tert-butylbenzene was used in the synthesis of 4-tert-butyl-phenylboronic acid1, 1-deoxy analogs of CP-47,497 (n = 0 to 7) and 1-deoxy analogs of CP-55,940 (n = 0 to 7). It undergoes lithium-bromide exchange reactions with n-butyllithium and tert-butyllithium at 0°C in various solvents.
Solubility
Insoluble in water.
Notes
Store in cool dry place. Keep away from oxidizing agents.
1-Bromo-4-tert-butylbenzene was used in the synthesis of 4-tert-butyl-phenylboronic acid1, 1-deoxy analogs of CP-47,497 (n = 0 to 7) and 1-deoxy analogs of CP-55,940 (n = 0 to 7). It undergoes lithium-bromide exchange reactions with n-butyllithium and tert-butyllithium at 0°C in various solvents.
Solubility
Insoluble in water.
Notes
Store in cool dry place. Keep away from oxidizing agents.
RUO – Research Use Only
General References:
- Michael Palucki.; Stephen L. Buchwald. Palladium-Catalyzed α-Arylation of Ketones.J. Am. Chem. Soc. 1997, 119 (45),11108-11109 .
- Jens Åhman.; John P. Wolfe.; Malisa V. Troutman.; Michael Palucki.; Stephen L. Buchwald. Asymmetric Arylation of Ketone Enolates.J. Am. Chem. Soc. 1998, 120 (8), 1918-1919 .