Search Thermo Fisher Scientific
Thermo Scientific Chemicals
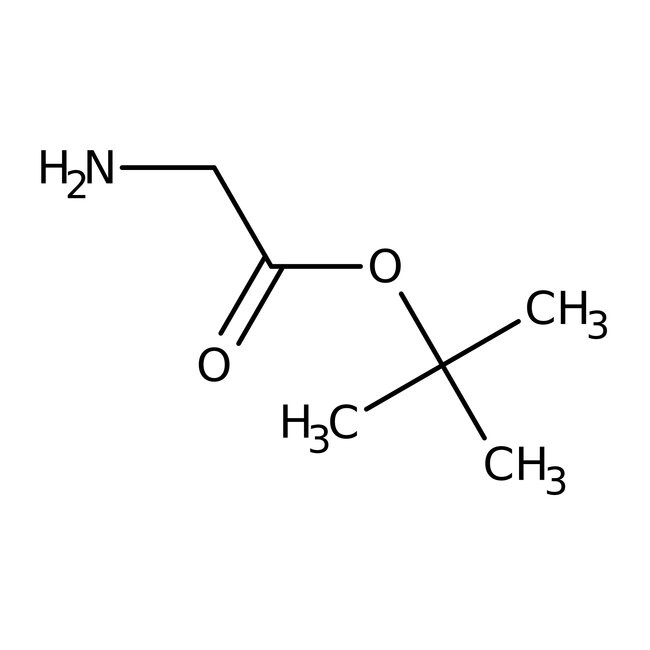
Glycine tert-butyl ester, 97%, Thermo Scientific ChemicalsCatalog number L16258.06
also known as L16258-06
Price (EUR)/ Each
217,80
Online exclusive
242.00 Save 24,20 (10%)
-
Quantity:
5 g
Price (EUR)/ Each
217,80
Online exclusive
242.00 Save 24,20 (10%)
Glycine tert-butyl ester, 97%, Thermo Scientific Chemicals
Catalog numberL16258.06
Price (EUR)/ Each
217,80
Online exclusive
242.00 Save 24,20 (10%)
-
Chemical Identifiers
CAS6456-74-2
IUPAC Nametert-butyl 2-aminoacetate
Molecular FormulaC6H13NO2
InChI KeySJMDMGHPMLKLHQ-UHFFFAOYSA-N
SMILESCC(C)(C)OC(=O)CN
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to yellow
Assay (GC)≥95.0%
FormLiquid
Refractive Index1.4215-1.4265 @ 20°C
Glycine tert-butyl ester is used in the preparation of Schiff base by reacting with benzophenone. The resultant Schiff base undergoes asymmetric alkylation with arylmethyl bromides in the presence of O-allyl-N-(9-anthracenylmethyl)cinchonidinium bromide as chiral phase transfer catalyst to give guanidine-containing pentacyclic compound.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Glycine tert-butyl ester is used in the preparation of Schiff base by reacting with benzophenone. The resultant Schiff base undergoes asymmetric alkylation with arylmethyl bromides in the presence of O-allyl-N-(9-anthracenylmethyl)cinchonidinium bromide as chiral phase transfer catalyst to give guanidine-containing pentacyclic compound.
Solubility
Miscible with acetone and methanol. Slightly miscible with ehtanol. Immiscible with water.
Notes
Air sensitive. Store in a cool place. Incompatible with strong oxidizing agents.
Glycine tert-butyl ester is used in the preparation of Schiff base by reacting with benzophenone. The resultant Schiff base undergoes asymmetric alkylation with arylmethyl bromides in the presence of O-allyl-N-(9-anthracenylmethyl)cinchonidinium bromide as chiral phase transfer catalyst to give guanidine-containing pentacyclic compound.
Solubility
Miscible with acetone and methanol. Slightly miscible with ehtanol. Immiscible with water.
Notes
Air sensitive. Store in a cool place. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Lou, S.; Ramirez, A.; Conlon, D. A. Catalytic syn-Selective Direct Aldol Reactions of Benzophenone Glycine Imine with Aromatic, Heteroaromatic and Aliphatic Aldehydes. Adv. Synth. Catal. 2015, 357 (1), 28-34.
- Peng, Z.; McLuckey, S. A. C-terminal peptide extension via gas-phase ion/ion reactions. Int. J. Mass Spectrom. 2015, 391, 17-23.