Search Thermo Fisher Scientific
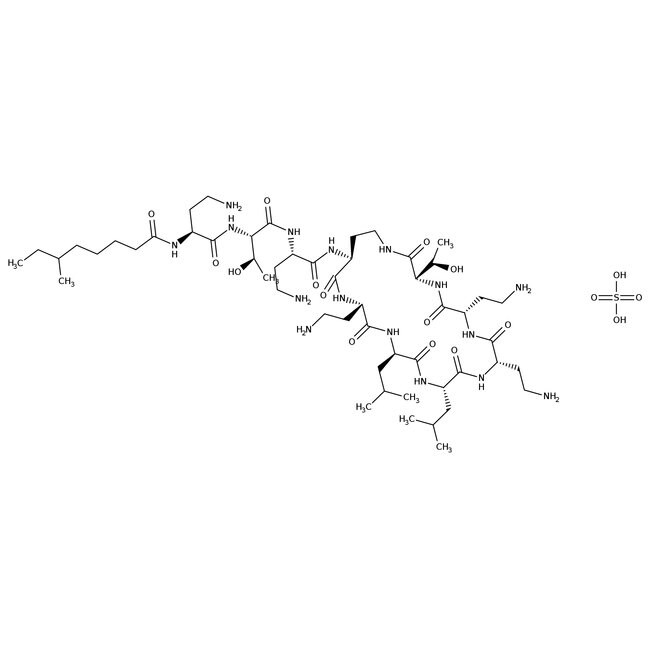
Colistin sulfate salt
| Catalog Number | Quantity |
|---|---|
| 455392500 | 250 mg |
| 455390010 | 1 g |
General description
• Colistin Sulfate salt is a stable, cationic, water-soluble, white powder composed of over 30 components
• Originally isolated from B. polymyxa, it inhibits the growth of the Gram-negative (e.g. E. coli, P. aeruginosa, P. fluorescens, and S. enterica), and Gram-positive bacteria (e.g. L. lactis, P. polymyxa, P. acidilactici, and S. aureus) by permeabilizing the bacterial cell membrane
Applications
• Colistin Sulfate has potent anti-endotoxin activity rendered through its ability to bind lippopolysaccharides that make up bacterial endotoxins
• This compound is often used in the management of multi-drug resistant Gram-negative infections
Literature References:
Falagas, M. E.; Kasiakou, S. K.; Saravolatz, L. D. Colistin: The Revival of Polymyxins for the Management of Multidrug-Resistant Gram-Negative Bacterial Infections Clinical Infectious Diseases. 2005, 40 (9), 1333–1341.
Suzuki, T.; Inouye, H.; Fujikawa, K.; et al. Studies on the chemical structure of colistin. I. Fractionation, molecular weight determination, amino acid and fatty acid composition. J. Biochem. 1963, 54(1), 25-33.
Naghmouchi, K.; Hammami, R.; Fliss, I.; et al. Colistin A and colistin B among inhibitory substances of Paenibacillus polymyxa JB05-01-1. Arch. Microbiol. 2012, 194(5), 363-370.
Mitsugui, C.S.; Tognim, M.C.B.; Cardoso, C.L.; et al. In vitro activity of polymyxins in combination with β-lactams against clinical strains of Pseudomonas aeruginosa. Int. J. Antimicrob. 2011, 38(5), 447-450.
Documents & Downloads
Safety Data Sheets
Safety and Handling
Classification of the substance or mixture
CLP classification - Regulation(EC) No 1272/2008
Label Elements
Signal Word
Danger
Hazard Statements
H301 - Toxic if swallowed
Precautionary Statements
P264 - Wash face, hands and any exposed skin thoroughly after handling
P301 + P310 - IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician