Search Thermo Fisher Scientific
Thermo Scientific Chemicals
Glyoxal dimethyl acetal, 60% aq. soln., Thermo Scientific ChemicalsCatalog number L18490.22
also known as L18490-22
Price (EUR)/ Each
56,07
Online exclusive
62.30 Save 6,23 (10%)
-
Quantity:
100 g
Price (EUR)/ Each
56,07
Online exclusive
62.30 Save 6,23 (10%)
Glyoxal dimethyl acetal, 60% aq. soln., Thermo Scientific Chemicals
Catalog numberL18490.22
Price (EUR)/ Each
56,07
Online exclusive
62.30 Save 6,23 (10%)
-
Chemical Identifiers
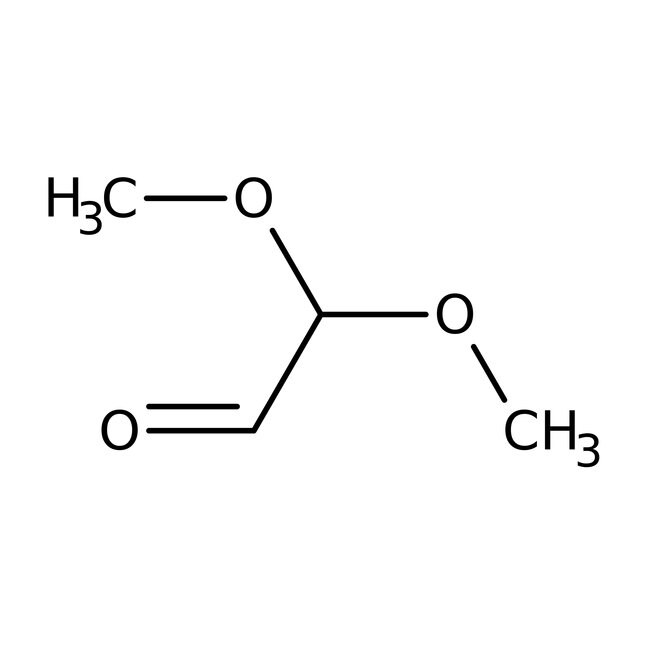
CAS51673-84-8
IUPAC Name2,2-dimethoxyacetaldehyde
Molecular FormulaC4H8O3
InChI KeyOGFKTAMJLKHRAZ-UHFFFAOYSA-N
SMILESCOC(OC)C=O
View more
Specifications Specification Sheet
Specification Sheet
Refractive Index1.4105-1.4150 @ 20?C
Appearance (Color)Clear colorless to pale yellow
Assay (Titration)≥58.0%
Identification (FTIR)Conforms
Free acid (titration)≤1.0%
View more
Glyoxal dimethyl acetal is an important raw material and intermediate used in organic synthesis, pharmaceuticals agrochemicals and dyestuffs.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Glyoxal dimethyl acetal is an important raw material and intermediate used in organic synthesis, pharmaceuticals agrochemicals and dyestuffs.
Solubility
Fully miscible in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are opened must be carefully resealed and kept upright to prevent leakage. Incompatible with oxidizing agents.
Glyoxal dimethyl acetal is an important raw material and intermediate used in organic synthesis, pharmaceuticals agrochemicals and dyestuffs.
Solubility
Fully miscible in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are opened must be carefully resealed and kept upright to prevent leakage. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Pacsu, E.; Trister, S. M.; Green, J. W. The Acetone Derivatives of the Mercaptals of Some Monosaccharides. IV. 1 The 4, 5-Monoacetone Derivative of the Dibenzylmercaptal and of the Dimethylacetal of d-Galactose. Journal of the American Chemical Society. 1939, 61 (9), 2444-2448.
- Laurent Soulère.; Yves Queneau.; Alain Doutheau. Kissick. An expeditious synthesis of 4-hydroxy-2(E)-nonenal (4-HNE), its dimethyl acetal and of related compounds. Chemistry and Physics of Lipids. 2007, 150 (2), 239-243.