Search Thermo Fisher Scientific
- Order Status
- Quick Order
-
Don't have an account ? Create Account
Search Thermo Fisher Scientific
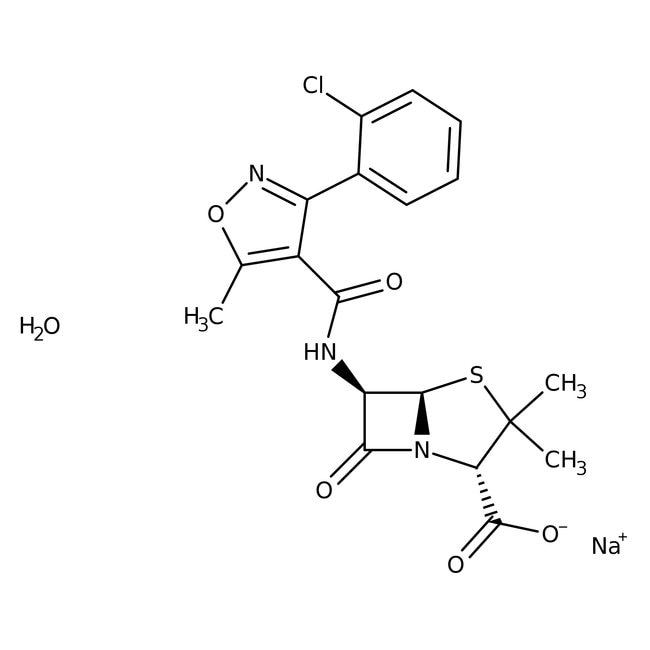

General Description
• Cloxacillin sodium salt monohydrate is a chlorinated derivative of oxacillin that shows β-lactamase-resistant antibiotic activity
• This compound inhibits the last stage of bacterial cell wall synthesis by binding to penicillin-binding proteins (PBPs), which results in cell lysis, that is mediated by bacterial cell wall autolytic enzymes
Applications
• Cloxacillin sodium salt monohydrate targets gram-positive bacteria, and is effective against staphylococci that produce β-lactamase
• It acts in vitro against both penicillin gram-resistant and gram-sensitive staphylococci, certain streptococci, and pneumococci
• This compound is commonly used in vivo and in vitro studies as AmpC β-lactamase inhibitor in combination with β-lactam antibiotics