Search Thermo Fisher Scientific
Thermo Scientific Chemicals
Trimethylene oxide, 97%, Thermo Scientific Chemicals
化學識別
IUPAC Nameoxetane
Molecular FormulaC3H6O
InChI KeyAHHWIHXENZJRFG-UHFFFAOYSA-N
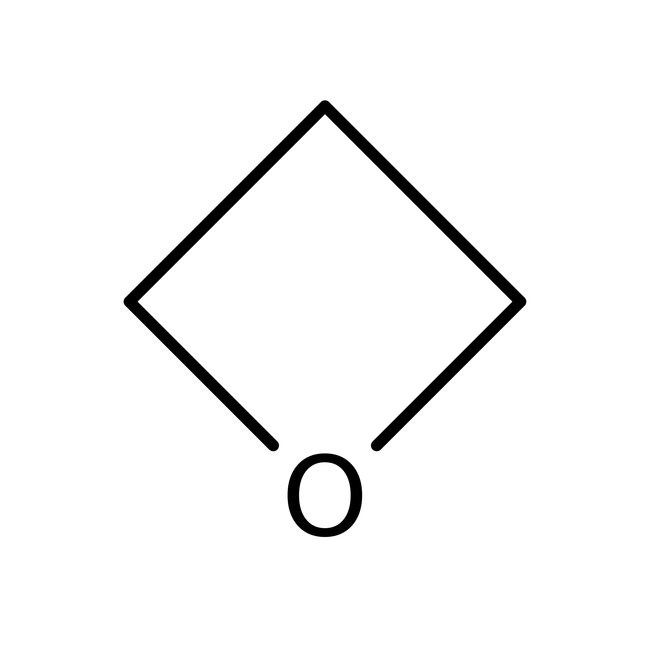
SMILESC1COC1
Molecular Weight (g/mol)58.08
檢視更多
規格 規格表
規格表
Assay (GC)≥96.0%
Refractive Index1.3900-1.3950 @ 20?C
FormLiquid
Appearance (Color)Clear colorless
Trimethylene oxide useful for the synthesis of 3-substituted propanols by reaction with Grignards or organolithiums in the presence of CuBr.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Trimethylene oxide useful for the synthesis of 3-substituted propanols by reaction with Grignards or organolithiums in the presence of CuBr.
Solubility
Soluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are opened must be carefully resealed and kept upright to prevent leakage. Recommended storage temperature is 2 - 8°C. It is hygroscopic in nature and sensitive to air. Store under inert gas.
Trimethylene oxide useful for the synthesis of 3-substituted propanols by reaction with Grignards or organolithiums in the presence of CuBr.
Solubility
Soluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are opened must be carefully resealed and kept upright to prevent leakage. Recommended storage temperature is 2 - 8°C. It is hygroscopic in nature and sensitive to air. Store under inert gas.
RUO – Research Use Only
General References:
- Scott Searles. The Reaction of Trimethylene Oxide with Grignard Reagents and Organolithium Compounds. J. Am. Chem. Soc. 1951, 73 (1), 124-125.
- Fouad Fleyfel.; J. Paul Devlin. FT-IR spectra of 90 K films of simple, mixed, and double clathrate hydrates of trimethylene oxide, methyl chloride, carbon dioxide, tetrahydrofuran, and ethylene oxide containing decoupled water-d2. J. Phys. Chem. 1988, 92 (3), 631-635.
- 3-Carbon fragment, useful for the synthesis of 3-substituted propanols by reaction with Grignards: J. Am. Chem. Soc., 73, 124 (1951), or organolithiums in the presence of CuBr: Tetrahedron Lett., 1095 (1976).
- Reaction with K metal: 18-crown-6 complex provides a one pot metallation reaction yielding a comparatively stable product for use in further metallation reactions: J. Chem. Soc., Chem. Commun., 1513 (1991).