Search Thermo Fisher Scientific
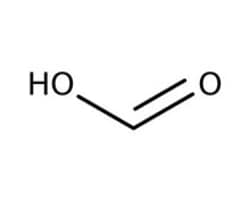
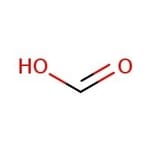
Formic Acid
Formic acid is the simplest carboxylic acid that is organic, flammable, and corrosive. It can be used as a mobile phase component in HPLC, a preservative and antibacterial agent in agriculture, and a hydride ion source in synthetic organic chemistry.
Products (4)
Learn More (27)
Documents & Support
(1188)4 Products
Filter
This Thermo Scientific Chemicals brand product was originally part of the Acros Organics product portfolio. Some documentation and label information may refer to the legacy brand. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to...
This Thermo Scientific Chemicals brand product was originally part of the Acros Organics product portfolio. Some documentation and label information may refer to the legacy brand. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to...
Used as a hydrogen source, as a reducing agent and is used in tanning and dye fixing. This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand.
It is principally used as a preservative antibacterial agent in livestock feed, in dying textiles, tanning leather and electroplating. In the poultry industry, it is sometimes added with feed to kill E. coli bacteria. It acts as a reducing agent for the catalytic reduction of nitrate in water.
Learn More (27)
View all
The most commonly used solvents or solvent blends include LC-MS grade water and acetonitrile, with ion pairing agents such as trifluoroacetic acid (TFA), formic acid (FA) or heptafluorobuteric acid (HFBA). These solvents are available as single component formulations or convenient blends.
Structure search Chem dex search Element search, Acids are molecules or ions that act as proton or hydrogen ion donors (Brønsted-Lowry acid) in non-aqueous solutions or can accept an electron pair in water (Lewis acid). Organic acids are typically weaker than inorganic acids.
Documents & Support (1188)
View all
The final concentration of formic acid in the sample that I am loading on a Bis-Tris gel will be 5%. Will this work?
Does the Small Molecule Suitability Standard need to be diluted?