Search Thermo Fisher Scientific
Cell and Gene Therapy virtual event on demand

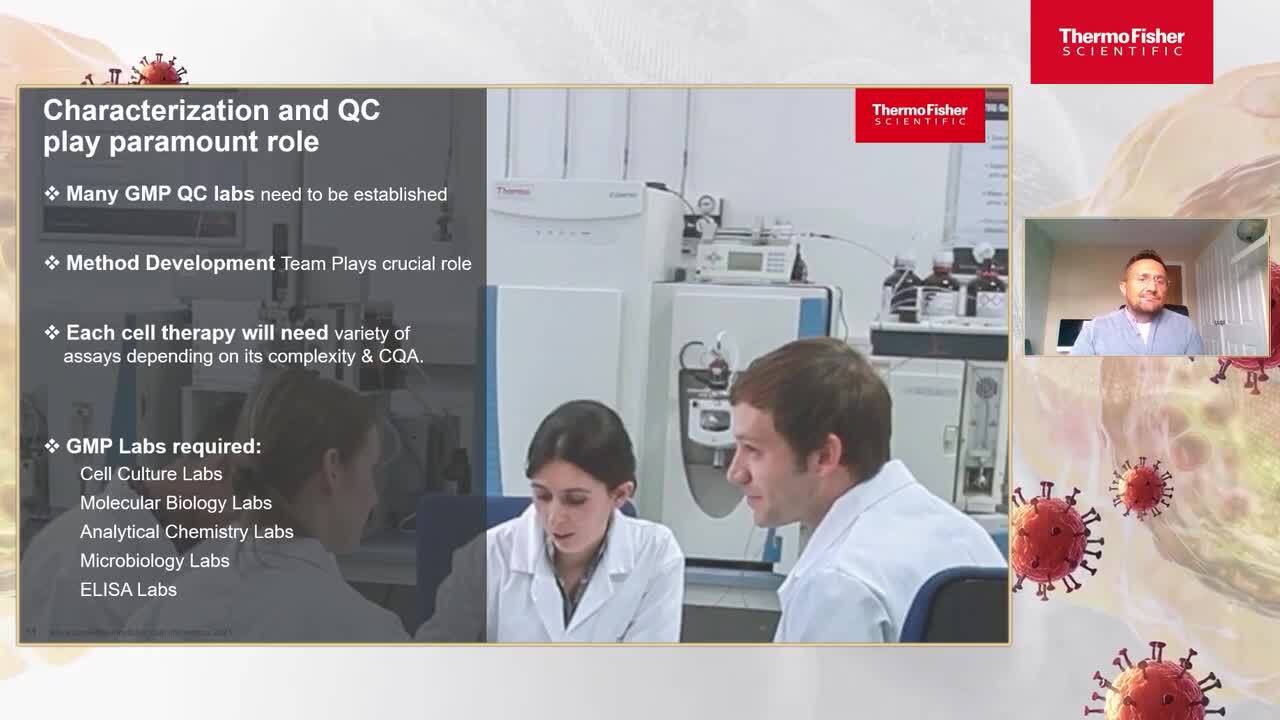
Day 1 Overcoming challenges in GMP Manufacturing
GMP compliance—Ongoing challenges for Advanced Therapy Medicinal Products (ATMPs)
In this session, our speakers define GMP compliance for ATMPs, starting from the aspects of manufacturing and operational processes.
Kris Wronski
Cell Culture Applications Scientist,
EMEA Cell Culture
Thermo Fisher Scientific
Thermo Scientific Cell Therapy Systems (CTS): Streamline the path from discovery to cure
Learn more about how Thermo Scientific CTS solutions can provide a proven choice for clinical stem cell therapy and immunotherapy research to transition your cell therapy to the clinic with confidence.
Matt Newsome
Commercial Manager
Thermo Fisher Scientific
Interactive panel discussion - How do companies overcome challenges in GMP
Hear directly from thought leaders in the field about how they approached common pain points while developing and producing cell and gene therapies in a GMP setting.
César Trigueros Fernandez
Chief Scientific Officer
Viralgen
Professor Mark Lowdell
Director of Centre for Cell, Gene & Tissue Therapeutics
Royal Free Hospital
Dimitrios Kouroupis
MSc, PhD Assistant Professor, Department of Orthopaedics
University of Miami, Miller School of Medicine
Day 2 Streamlining the transition from Process Development to Manufacturing
Raw Material Considerations for Cell and Gene Therapy
In this talk, we discuss the aspects of raw material and supplier selection considerations to help ensure a successful transition from clinical research to commercialization.
Sandy Kuliogowski
Senior Manager, Regulatory Affairs
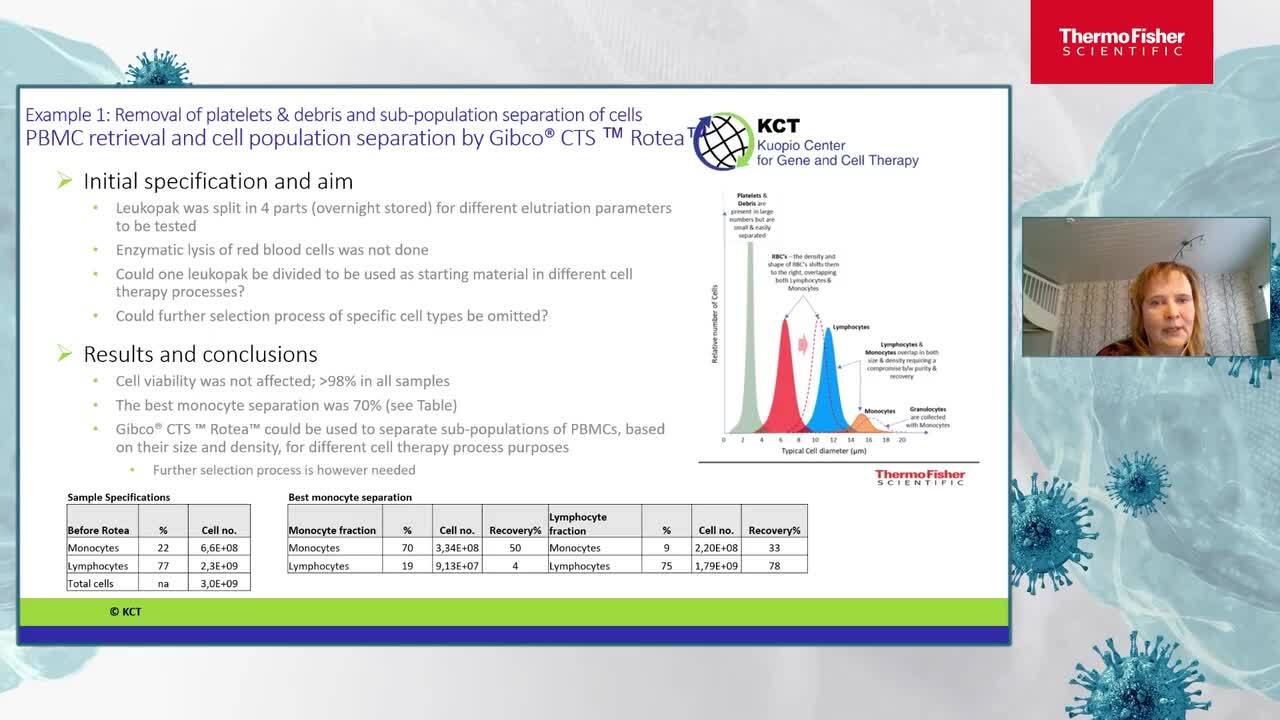
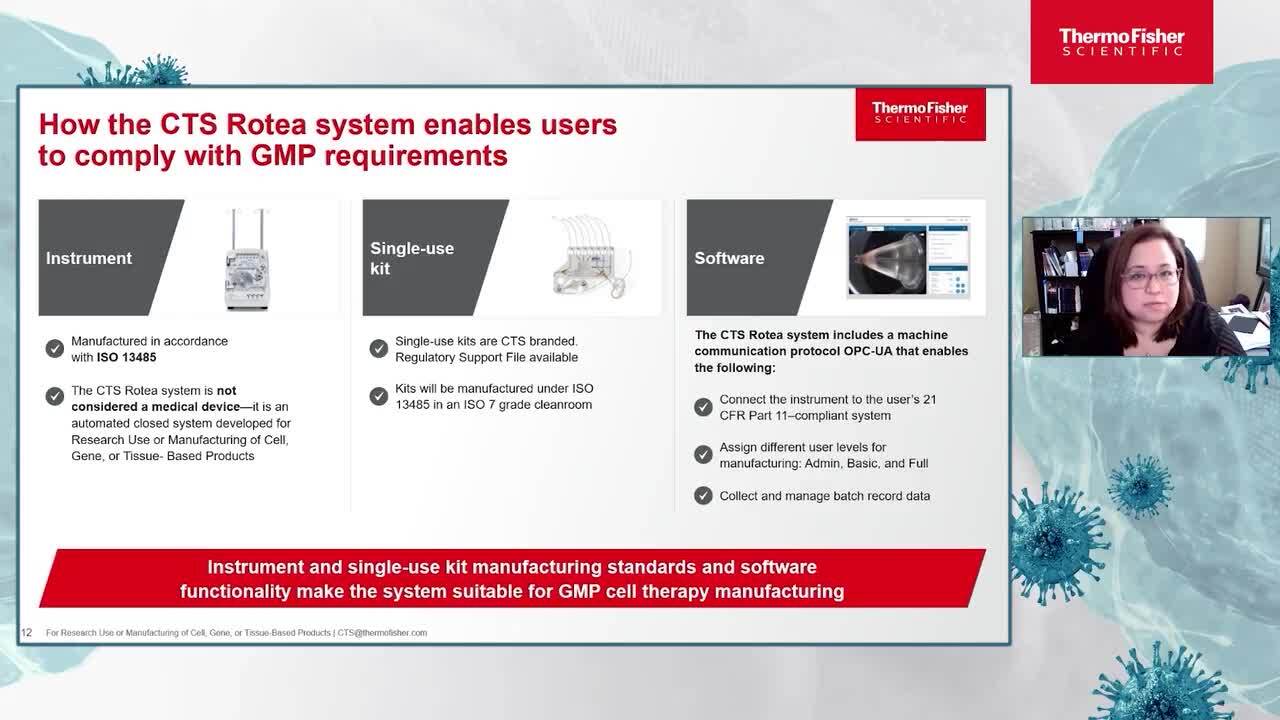
CTS Rotea provides flexible and functionally-closed processing of Cell Therapy products
Learn more about the current research at Kuopio Center for Gene and Cell Therapy (KCT). How they are aiming to close and automate their current process, the challenges they faced in this process. Finally, review the solutions that were evaluated and why they choose the CTS Rotea system to streamline their process.
Katja Sirviö
Cell Therapy Process Development Manager
Kuopio Centre for Gene and Cell Therapy
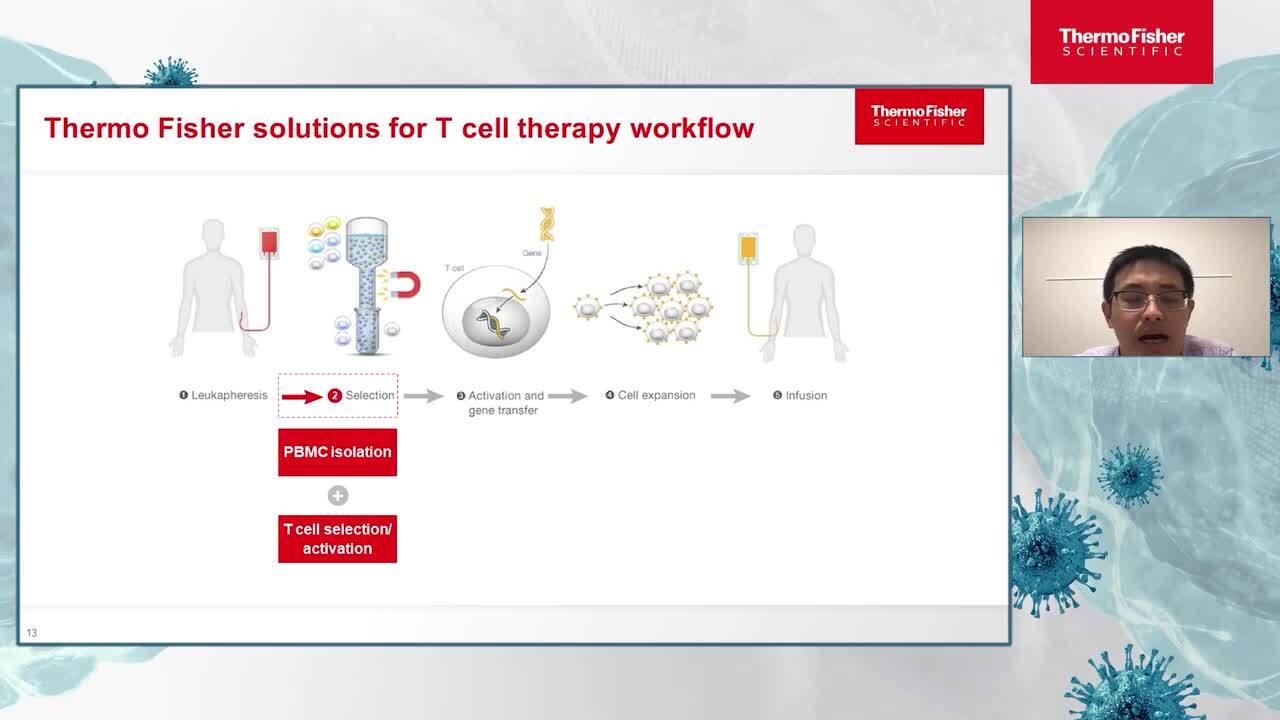
Closed manufacturing workflow for CAR T cell therapy development
In this talk, learn more about the advancements in Cell-based chimeric antigen receptor (CAR) T cell therapies.
Yongchang Ji, PhD
Manager, Cell and Gene Therapy,
Thermo Fisher Scientific
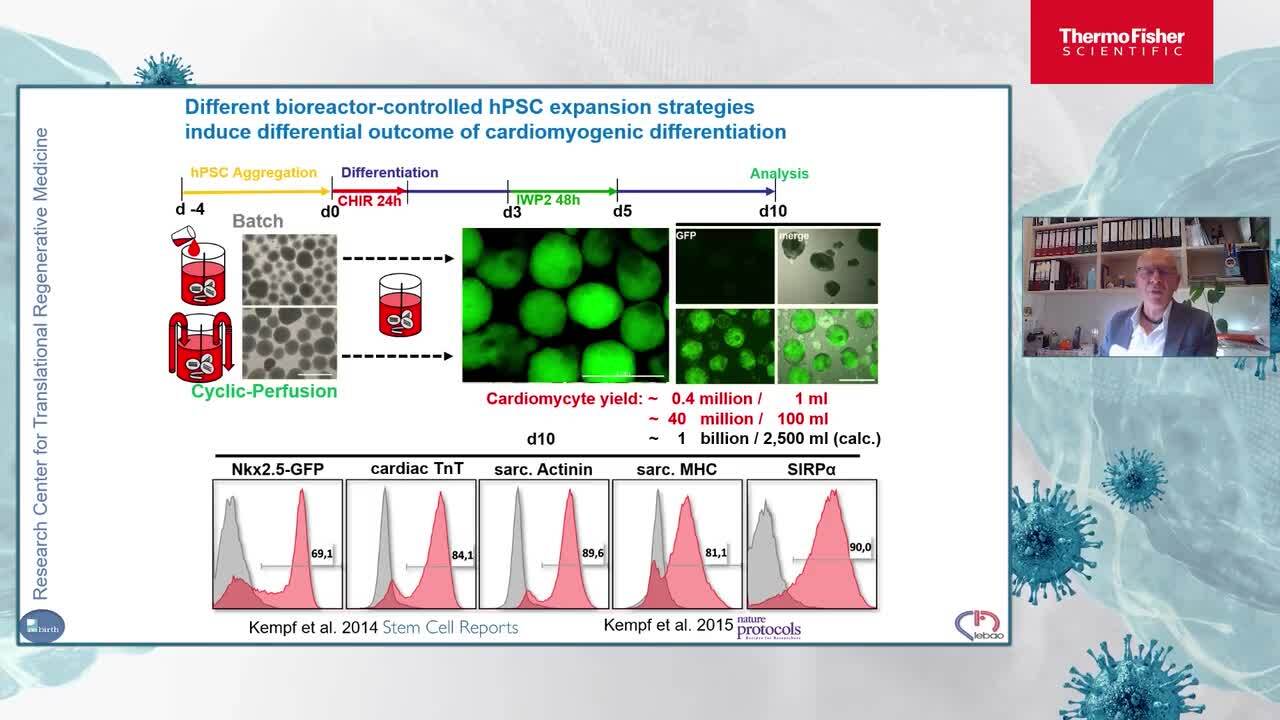
Advanced bioprocessing of hPSC-cardiomyocytes for heart repair
This talk will focuses on advanced hPSCs bioprocessing in fully controlled stirred tank bioreactors (STBR) including directed differentiation into specific lineages in particular hPSC-derived cardiomyocytes and potential strategies for their clinical translation by functional testing in preclinical animal models.
Dr. Robert Zweigerdt, PhD
Principal Investigator
Leibniz Research Laboratories for Biotechnology and Artificial Organs
Versatility of the Gibco CTS Rotea Counterflow Centrifugation System
In this talk, we discuss the value of moving towards modular and automated, closed-system technologies designed to enable scalable and cost-effective manufacturing for cell and gene therapies.
Mary Ann F. Santos
Senior Business Development Manager, Cell and Gene Therapy
Thermo Fisher Scientific
Day 3 Accelerate scale-up in upstream processing for Gene Therapy development
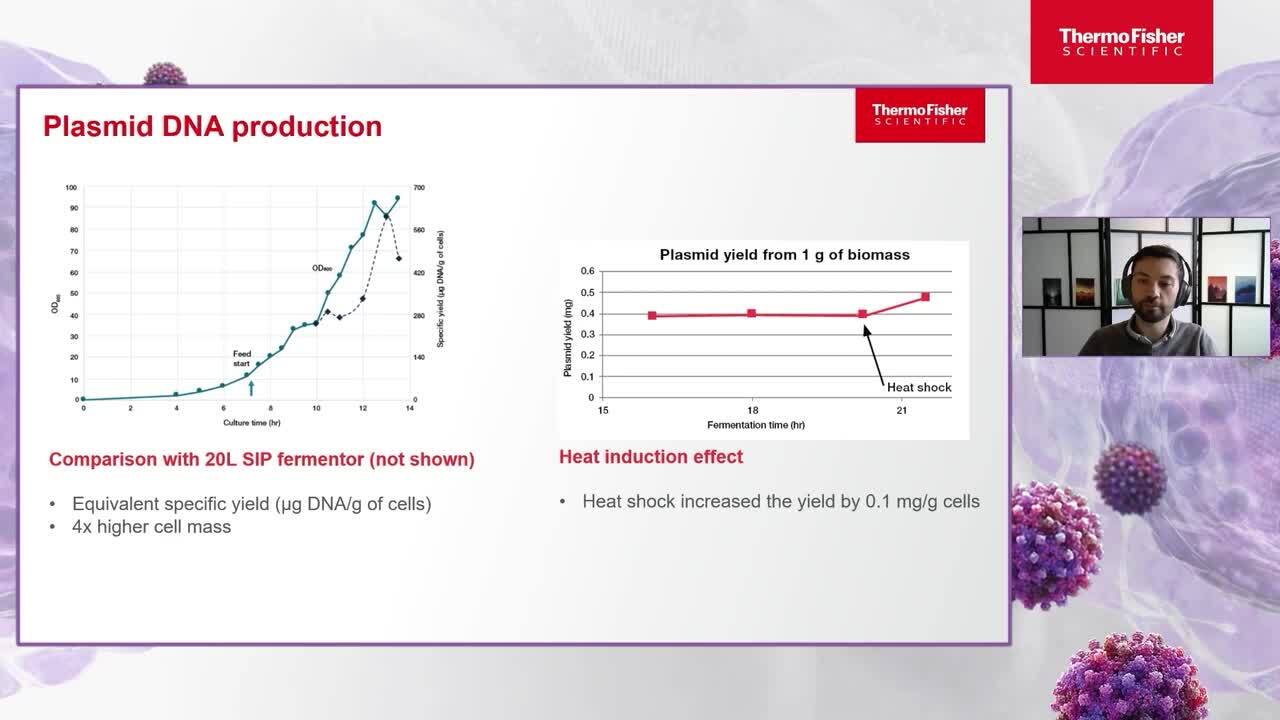
Streamlining plasmid DNA production for scale-up
In this session, we highlight our innovative solutions to optimize plasmid DNA production for your gene therapy development.
Alex Boscolo
Field Application Specialist Single-use Technologies
Thermo Fisher Scientific
Massimo Ferretti, PhD
Field Application Scientist, Gibco Bioproduction Cell Culture,
Thermo Fisher Scientific
Scalable, High-Titer, Simplified AAV Production in Gene Therapy
Watch as Thermo Fisher Scientific R&D scientist, Chao Yan Liu, discusses the challenges in Adeno-associated virus production. Hear more about one of our latest innovations in this space with the introduction of Gibco AAV-Max Helper-Free AAV Production System – which is a cost effective system for scalable, high-titer, simplified AAV production.
Chao Yan Liu, M.D.
Senior Manager, Cell Biology
Thermo Fisher Scientific
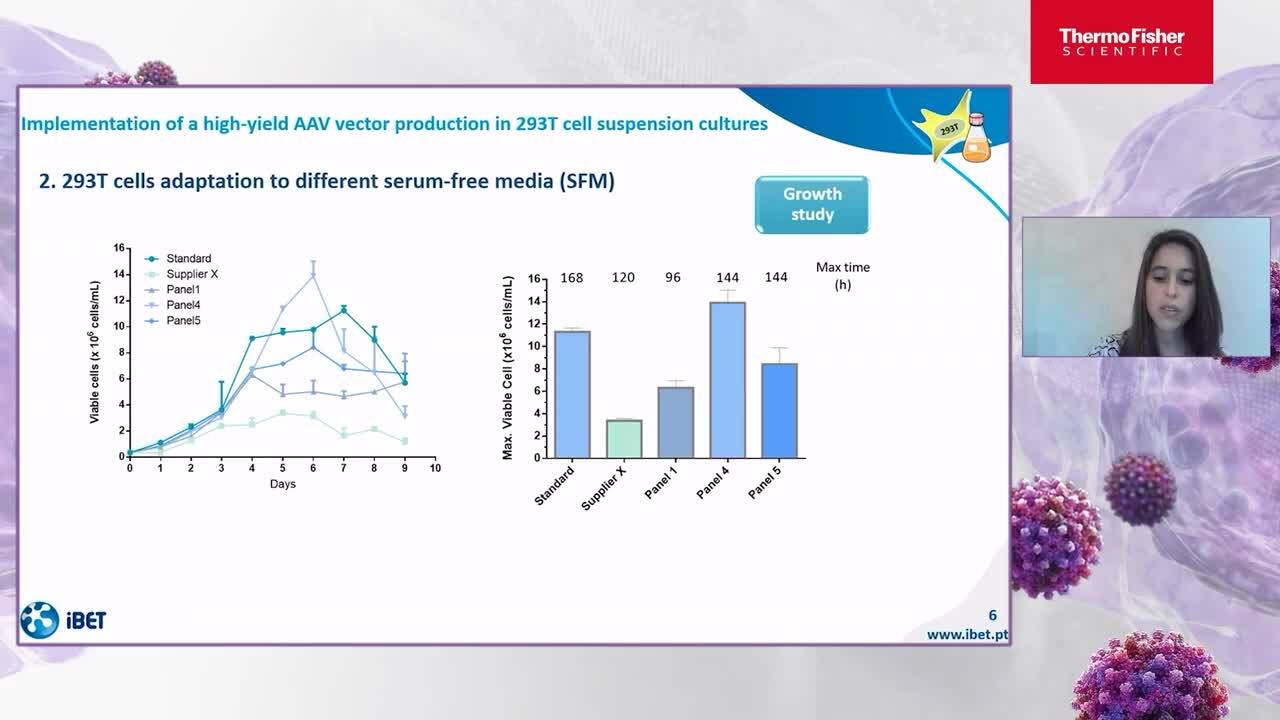
Benefits of using a media panel to address the diversity of HEK293 cell lines
In this talk, we’ll discuss the benefits of using a media panel to address the diversity of HEK293 cell lines using the Gibco Viral Vector HEK Media Panel. Research Biotech Organization, iBET will also join us to discuss their experience using the HEK293 media panel.
Sofia Fernandes PhD
Senior Research Associate, Cell Line Development & Molecular Virology Lab,
iBET
Céline Martin PhD
Global Product Manager Thermo Fisher Scientific
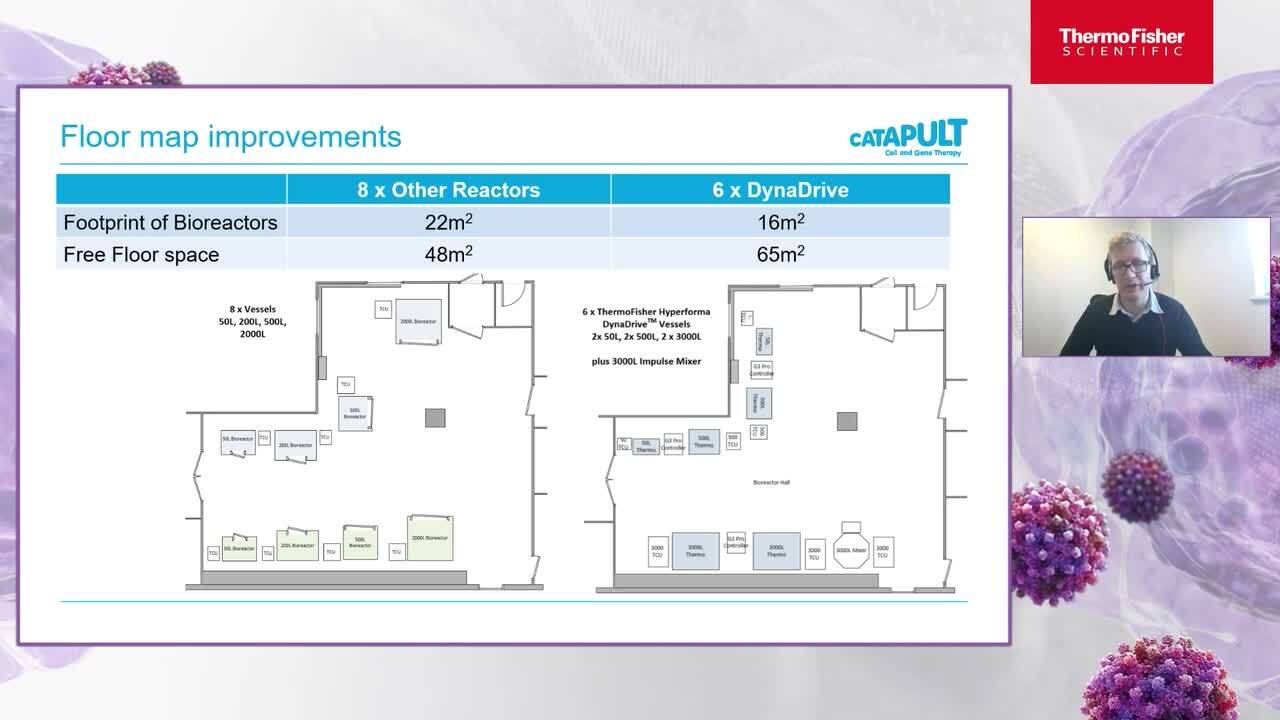
Keynote speaker: Addressing challenges in viral vector manufacturing
Watch this on-demand session and hear from global CDMO, Catapult as they discuss innovations in Cell and Gene therapy and address the challenges in viral vector manufacturing.
Peter Dashwood
MS&T Lead at CGTC Braintree
Catapult
Day 4 Accelerate scale-up in downstream processing for Gene Therapy development
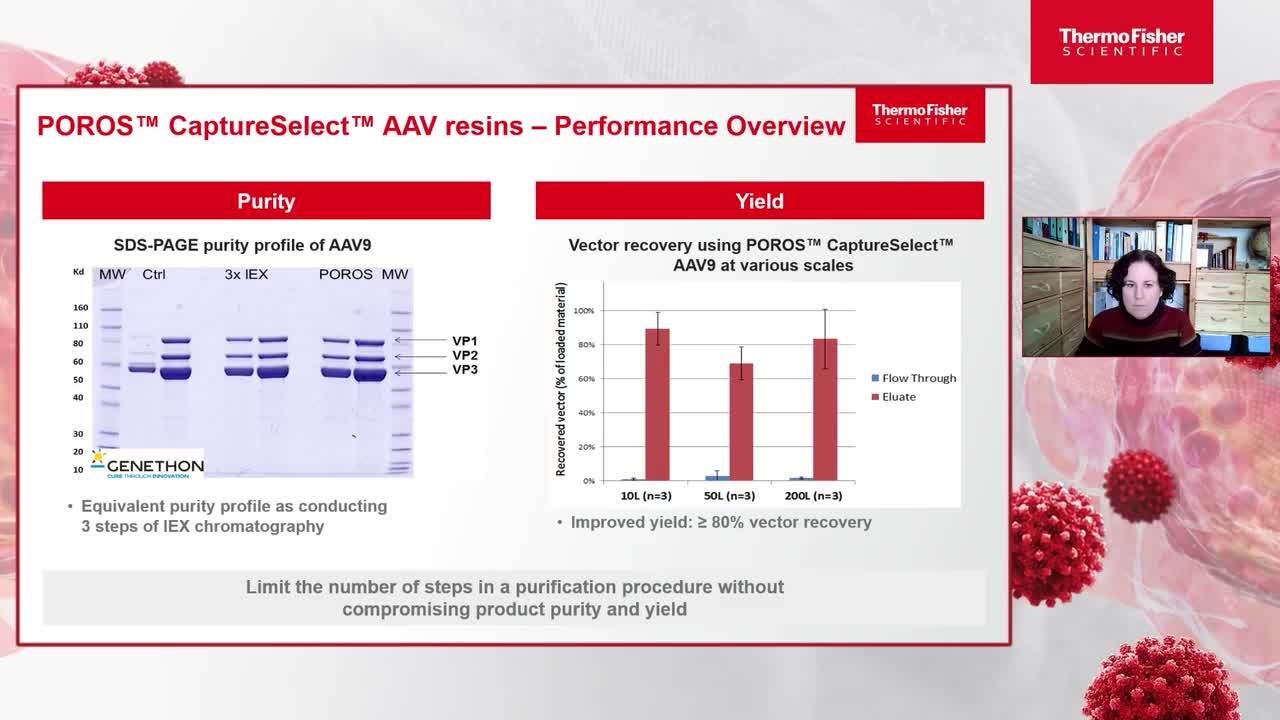
Chromatography solutions for AAV downstream processing
In this talk, learn more about the benefits of Affinity chromatography in downstream processing for gene therapy development.
Anna Le Bris
Senior Application Specialist and Trainer
Thermo Fisher Scientific
Highly sensitive residual DNA quantitation for Gene therapy production
In this presentation, learn more about our resDNASEQ kits for the quantitation of residual host cell DNA which have been designed to meet the required regulatory expectations.
Nico Chow
Field Application Specialist
Thermo Fisher Scientific
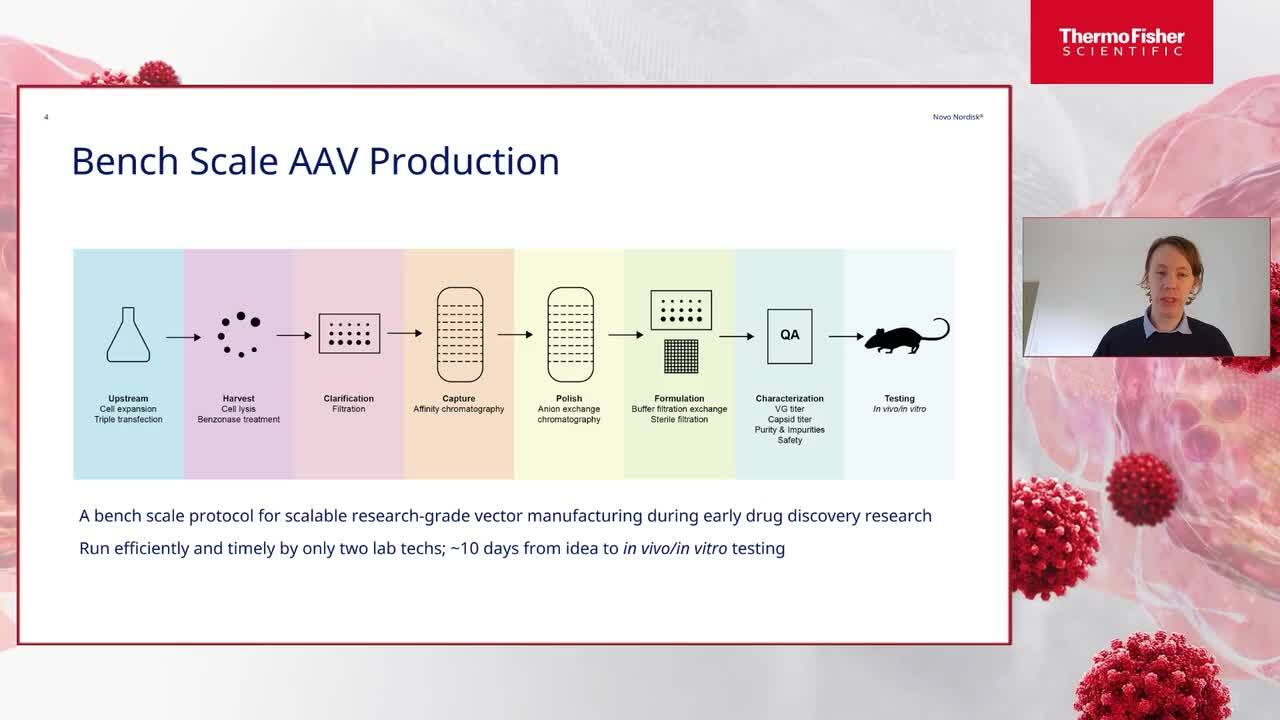
Implementing scalable vector production during early drug discovery research
Hear from Novo Nordisk as they discuss a bench scale production protocol for scalable research-grade vector production during early drug discovery research.
Toke Jost Isaksen, PhD
Senior Scientist, RNA and Gene Therapies, Global Research Technologies,
Novo Nordisk
Keynote speaker interview: The future of Gene Therapy
Now more than ever, we need to look to the future of Gene Therapy. Hear from global CDMO, Viralgen as they discuss new product innovations and address challenges faced in Gene Therapy manufacturing.
Javier García Cogorro
CEO
Viralgen