Search Thermo Fisher Scientific
Research and Clinical Diagnostic Tests and Controls
Browse our comprehensive selection of research and clinical dignostic tests and controls, including calibrators, reagents, drug monitoring and testing supplies, allergy and immunology testing kits, flu and virus detection tools, urine testing, drug screening, and allergen identification materials. Products may be suitable for clinical or research use - details are available at the product level.

Chemistry Controls, Calibrators and Reagents (665)

Other Controls and Reagents (272)

Drug Monitoring and Testing (98)

Allergy, Arthritis and Immunology Testing (53)

Flu and Virus Testing (47)
Urine Testing Reagents, Controls and Strips (5)
Point of Care Testing (1)
Products (1141)
Learn More (1779)
Documents & Support
(20)1141 Products
Filter
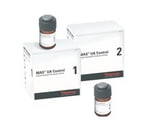
Monitor urine dipstick and microscopic components with Thermo Scientific™ MAS™ UA Controls, bi-level controls with improved room temperature storage capability. Liquid and ready-to-use, this urine control is assayed for most major reagents strips and strip systems.
Detect and differentiate Influenza A and B virus in clinical samples with Thermo Scientific™ IMAGEN™ Influenza Virus A and B Kit. This direct immunofluorescence assay utilizes species specific monoclonal antibodies to detect epitopes of Influenza virus glycoproteins and fusion proteins specific to...
Utilize the globally valued, highly accurate, and precise Thermo Scientific™ CEDIA™ Benzodiazepine Assay for very stable shelf life and lot-to-lot dependability. Applications are available for an array of clinical chemistry analyzers.
The Applied Biosystems TaqPath COVID-19 Diagnostic PCR Kit is a fast, highly sensitive multiplex diagnostic solution that contains the assays and controls needed for the real-time PCR detection of RNA from the SARS-CoV-2 virus in nasopharyngeal and anterior nasal specimens.
Determine quantity of carbon dioxide in serum in vitro Thermo Scientific™ Carbon Dioxide Reagents.
Quantify specific IgE antibodies with ImmunoCAP™ Occupational Allergen Components down to the molecular level, essential tools to aid in the diagnosis of allergic conditions. IgE antibodies appear in human serum and plasma as a result of sensitization to a specific allergen.
Assess the analytical performance of molecular test procedures, including the nucleic acid extraction step, for the quantitative and qualitative determination of viruses related to transplant disease with the Thermo Scientific™ AcroMetrix™ Transplant Virus Panels (RUO).
Thermo Scientific™ Quanti-Cult Plus™ Aspergillus brasiliensis ATCC™ 16404™ is supplied as a film of preserved microorganisms on the inside of the cap on a plastic vial.
Quantify specific IgE antibodies with ImmunoCAP™ Food Additive Allergens, essential tools to aid in the diagnosis of allergic conditions. IgE antibodies appear in human serum and plasma as a result of sensitization to a specific allergen.
Assess the performance of nucleic acid test procedures for the qualitative determination of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) nucleic acids in swab and urine patient specimens with Thermo Scientific™ AcroMetrix™ CT/NG Control.
Get relief from time-consuming and costly QC standard lot changes for up to 5 years with ready-to-use, liquid assayed Thermo Scientific™ MAS™ Liquimmune™ Immunoassay Controls. With a new 5-year shelf life, these multi-analyte controls simplify QC while monitoring immunoassay test procedures on most...
Thermo Scientific™ Quanti-Cult Plus™ Candida albicans ATCC™ 10231™ is supplied as a film of preserved microorganisms on the inside of the cap on a plastic vial.
This product has been discontinued. Consider individual strains found from the set: Mfr. Nos. R4603050 and R4605001. Thermo Scientific™ Culti-Loops™ BBL Crystal ID Systems Anaerobe Set contains quality control organisms supplied in Culti-Loops format.
Monitor day-to-day test variation, lot-to-lot performance of test kits, and operator variation with the Thermo Scientific™ AcroMetrix™ HCV Controls. These controls identify random or systematic errors in nucleic acid test procedures for the quantitative and qualitative determination of hepatitis C...
Estimate the precision and reproducibility of procedures for the detection of hepatitis B surface antigen (HBsAg), antibodies to hepatitis B core antigen (anti-HBc), antibodies to hepatitis C virus (anti-HCV), antibodies to human immunodeficiency virus type 1 group M and O (anti-HIV-1 group M and...
Learn More (1779)
View all
Genetic information is rapidly transforming the future of healthcare by enabling accurate and affordable diagnosis and prognosis, targeted treatments, and monitoring solutions. We are committed to providing reliable and easy-to-use solutions, services, and support to meet the demanding needs of your...
Together, we are moving medicine forward by bringing you advanced diagnostic tools. We are committed to providing reliable and easy-to-use instruments, solutions, services and support to meet the needs of today's diagnostic laboratories.
Documents & Support (20)
View all
What is the difference between TrypLE Express Enzyme, TrypLE Select Enzyme, and CTS TrypLE Select Enzyme?
Since the PureQuant assay technology is licensed-in from a third party, are there any restrictions on its use?