Search Thermo Fisher Scientific
Flu and Virus Testing
Flu and virus testing encompass reagents used to detect peptides or antibodies for the flu or other viruses. Products include tests for COVID, respiratory, influenza, mononucleosis, and human Metapneuomovirus viruses.

Respiratory Viral Testing (21)
COVID Testing Kits (12)

Influenza Testing (6)

Mononucleosis Testing (5)

Human Metapneumovirus Testing (2)

Multiplex COVID Testing Kits (1)
Products (47)
Learn More (274)
Documents & Support
(17)47 Products
Filter
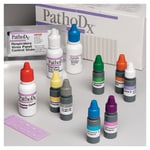
Use Thermo Scientific™ Remel™ PathoDx™ Respiratory Virus Panel Influenza A Reagent along with PathoDx™ Respiratory Virus Panel (RVP) kit to perform a direct immunofluorescence test for the qualitative detection of Influenza A in prepared patient specimens following growth in cell culture.
Use Thermo Scientific™ Remel™ PathoDx™ Respiratory Virus Panel (RVP) kit to perform direct immunofluorescence test for the qualitative detection of 7 common respiratory viruses (Respiratory Syncytial Virus [RSV], Influenza A, Influenza B, Parainfluenza viruses 1, 2, and 3, and Adenovirus) in...
Validate and monitor the performance of molecular diagnostic tests for detecting COVID-19, Flu A/B and RSV A/B using the AcroMetrix™ Multi-Analyte SARS-CoV-2 Control, Flu A/B, RSV A/B Control (RUO). This multiplex control can be used as a complete assay control starting from extraction to...
The Applied Biosystems TaqPath COVID-19 Diagnostic PCR Kit is a fast, highly sensitive multiplex diagnostic solution that contains the assays and controls needed for the real-time PCR detection of RNA from the SARS-CoV-2 virus in nasopharyngeal and anterior nasal specimens.
The TaqPath COVID-19, Flu A, Flu B Combo Kit includes the assays and controls for a multiplex real-time RT-PCR test for the qualitative detection and differentiation of RNA from SARS-CoV-2, influenza A, and influenza B viruses in nasopharyngeal and anterior nasal swabs from individuals suspected of...
Validate the test procedures of Thermo Scientific™ Xpect™ Remel™ Flu A and B test kits with Thermo Scientific Xpect Remel Flu A and B Control Swab. These kits contain positive and negative controls to monitor test performance.
Perform direct immunofluorescence test for the qualitative detection of Respiratory Syncytial Virus (RSV) in prepared direct patient specimens and following growth in cell culture using Thermo Scientific™ Remel™ PathoDX™ RSV kit.
Rapidly detect and differentiate Parainfluenza virus types 1, 2 and 3 antigens directly in nasopharyngeal aspirates or in cell culture preparations with Thermo Scientific™ IMAGEN™ Parainfluenza Virus Typing Kit.
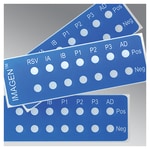
The IMAGEN™ Control Slides are for use as a control to the following tests: IMAGEN Adenovirus (K610011-2) IMAGEN Respiratory Syncyti al Virus (RSV) (K610211-2) IMAGEN Parainfluenza Group (K610311-2) IMAGEN Parainfluenza Typing 1, 2 and 3 (K610411-2) IMAGEN Influenza A and B Virus (K610511-2) IMAGEN...
Validate and monitor the performance of molecular diagnostic tests for detecting COVID-19 using the Thermo Scientific™ AcroMetrix™ Coronavirus 2019 (COVID-19) RNA Control (RUO). This is a positive control kit containing two concentrations: a Low Positive and Ultra-Low Positive concentration to avoid...
Rapidly detect Parainfluenza virus types 1, 2 and 3 antigens directly in nasopharyngeal aspirates or in cell culture preparations with Thermo Scientific™ IMAGEN™ Parainfluenza Group Kit. This direct immunofluorescence assay utilizes type-specific monoclonal antibodies to detect and identify...
Presumptively detect respiratory syncytical virus (RSV), influenza A and B virus, parainfluenza virus 1,2 & 3 and adenovirus in respiratory specimens with Thermo Scientific™ IMAGEN™ Respiratory Virus Screen Kit. The assay cannot differentiate between the viruses.
The Amplitude High‐Throughput Consumable Package 1 Reagent Kit contains reagents for 20,000 reactions. This package allows for streamlined ordering of reagents to efficiently address high-throughput testing needs.
Applied Biosystems TaqMan SARS-CoV-2 Control Dilution Buffer is used to dilute the TaqMan SARS-CoV-2 RNA Control (Cat. No. 956118), which is used with the TaqMan SARS-CoV-2 Pooling Assay Kit (Cat. No. A50790).
Learn More (274)
View all
Nucleic acids from the viral organisms identified by this test are generally detectable in NP and AN swab specimens during the acute phase of infection. The detection and identification of specific viral nucleic acids from individuals exhibiting signs and symptoms of respiratory tract infection are...
Regulatory agencies expect all biologics to undergo viral testing to reduce the risk of viral contamination. Sf-rhabdovirus, Mouse Minute Virus (MMV) and Vesivirus are all viral contaminants that are potential threats to drug development manufacturing processes.
Documents & Support (17)
View all
Instructions: Swine Influenza Virus RNA Test Kit - VetMAX-Gold SIV Detection Kit
Product Information Sheet: Swine Influenza Virus RNA Test Kit - VetMAX-Gold SIV Subtyping Kit