Search Thermo Fisher Scientific
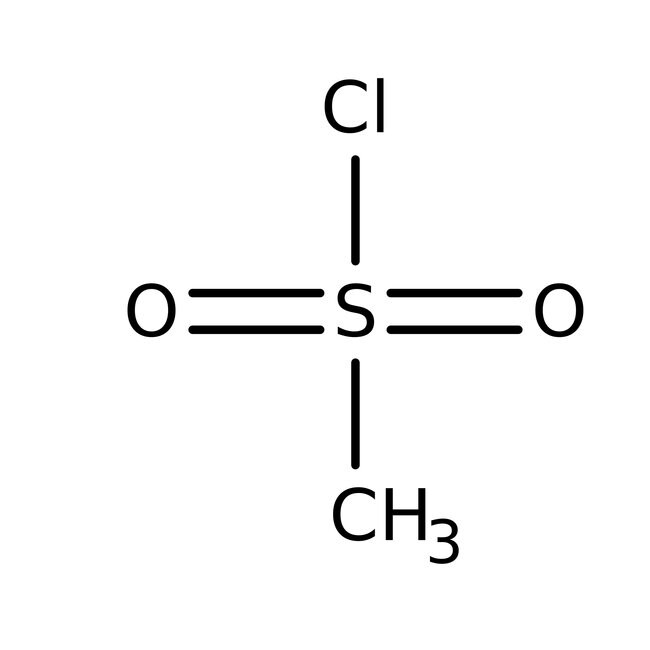
Methanesulfonyl chloride, 98%, Thermo Scientific Chemicals
化学物質識別子
仕様
概要
Methanesulfonyl chloride is used as a reagent for conversion of alcohols to mesylate esters such as methanesulfonate, which is an intermediate in substitution reactions, elimination reactions, reductions, and rearrangement reactions viz. Beckmann rearrangement. It is an electrophile and acts as a source of CH3SO2+ group. It is also used to prepare beta-chloro sulfones, methanesulfonamide and heterocyclic compounds containing five membered sultones.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Methanesulfonyl chloride is used as a reagent for conversion of alcohols to mesylate esters such as methanesulfonate, which is an intermediate in substitution reactions, elimination reactions, reductions, and rearrangement reactions viz. Beckmann rearrangement. It is an electrophile and acts as a source of CH3SO2+ group. It is also used to prepare beta-chloro sulfones, methanesulfonamide and heterocyclic compounds containing five membered sultones.
Solubility
Miscible with alcohol, ether and organic solvents. Immiscible with water.
Notes
Moisture sensitive. Store in a cool place. Incompatible with strong bases, oxidizing agents and alcohols. It is a lachrymator and reacts with water.