Search Thermo Fisher Scientific
Thermo Scientific Chemicals
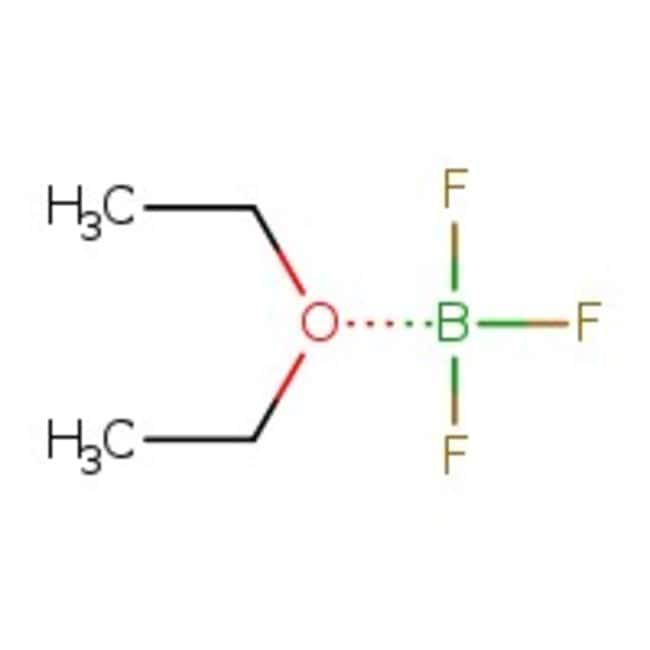
Boron trifluoride diethyl etherate, 98+%, Thermo Scientific Chemicals
製品番号(カタログ番号): A15275.AP
500 mL, Each
化学物質識別子
CAS
109-63-7
IUPAC Name
ethoxyethane; trifluoroborane
Molecular Formula
C4H10BF3O
InChI Key
KZMGYPLQYOPHEL-UHFFFAOYSA-N
SMILES
FB(F)F.CCOCC
仕様
Form
fuming liquid
Appearance (Color)
Colourless to pale brown
Assay from Supplier's CofA
≥47.0 to ≤49.5 % w/w (as BF3)
概要
Boron trifluoride diethyl etherate is used as a Lewis acid catalyst in Mukaiyama aldol addition, alkylation, acetylation, isomerization, dehydrations and condensation reactions. It is involved in the prepattion of polyethers in polymerization reactions. As a catalyst, it is used in the preparation of cyclopentyl- and cycloheptyl[b]indoles and other diborane. It is also used in sensitive neutron detectors in ionization chambers as well as monitoring radiation levels in earth’s atmosphere.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Boron trifluoride diethyl etherate is used as a Lewis acid catalyst in Mukaiyama aldol addition, alkylation, acetylation, isomerization, dehydrations and condensation reactions. It is involved in the prepattion of polyethers in polymerization reactions. As a catalyst, it is used in the preparation of cyclopentyl- and cycloheptyl[b]indoles and other diborane. It is also used in sensitive neutron detectors in ionization chambers as well as monitoring radiation levels in earth’s atmosphere.
Solubility
Miscible with ether and alcohol.
Notes
Moisture sensitive. Store in cool place. Recommended to use under inet atomosphere. Incompatible with metals, acids, bases, alcohols, alkali metals, oxidizing agents and water. Reacts violently with water.
Boron trifluoride diethyl etherate is used as a Lewis acid catalyst in Mukaiyama aldol addition, alkylation, acetylation, isomerization, dehydrations and condensation reactions. It is involved in the prepattion of polyethers in polymerization reactions. As a catalyst, it is used in the preparation of cyclopentyl- and cycloheptyl[b]indoles and other diborane. It is also used in sensitive neutron detectors in ionization chambers as well as monitoring radiation levels in earth’s atmosphere.
Solubility
Miscible with ether and alcohol.
Notes
Moisture sensitive. Store in cool place. Recommended to use under inet atomosphere. Incompatible with metals, acids, bases, alcohols, alkali metals, oxidizing agents and water. Reacts violently with water.
RUO – Research Use Only
図
ドキュメントおよびダウンロード
証明書
ロット番号または部分ロット番号で検索
よくあるご質問(FAQ)
引用および参考文献
Search citations by name, author, journal title or abstract text