Search Thermo Fisher Scientific
Thermo Scientific Chemicals
Phenol, detached crystals, 99+%, Thermo Scientific Chemicals化学物質識別子
CAS108-95-2
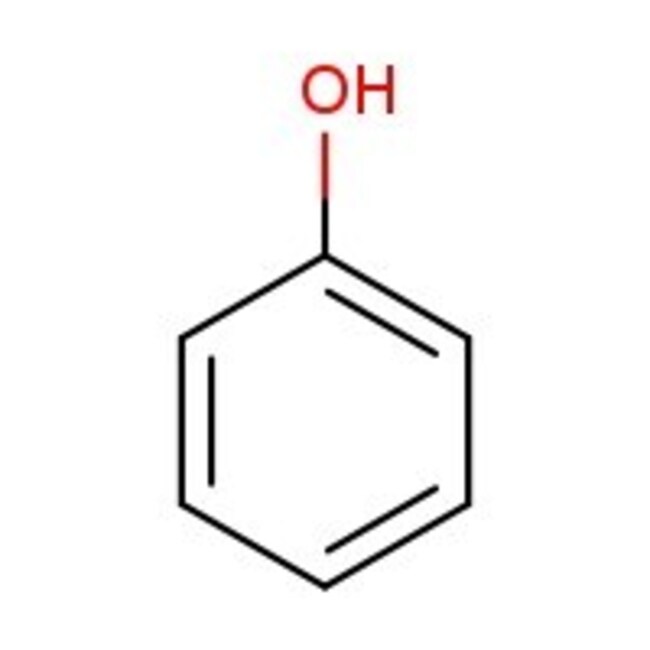
IUPAC Namephenol
Molecular FormulaC6H6O
InChI KeyISWSIDIOOBJBQZ-UHFFFAOYSA-N
SMILESOC1=CC=CC=C1
さらに表示
仕様 スペックシート
スペックシート
Appearance (Color)White
Identification (FTIR)Conforms
Assay (GC)≥99.0%
FormCrystals or powder or crystalline powder or fused solid
Melting Point (clear melt)38.0-44.0?C
Phenol is used as a precursor for cyclohexanone, plastics, nonionic detergents and pharmaceutical drugs like aspirin. It acts as an anesthetic in chloraseptic. It reacts with acetone to get bisphenol-A, which is used in the synthesis of poly carbonates and epoxide resins. It is also used in the manufacture of synthetic resins, dyes, pharmaceuticals, synthetic tanning agents, perfumes, lubricating oils and solvents. In molecular biology, it is used in the extraction of nucleic acid from tissues by using liquid/liquid phenol-chloroform extraction technique. It is an active component of paint strippers, which is used for the removal of epoxy and polyurethane. It is also used in the preparation of cosmetics, hair colorings and skin lightening preparations. In the field of medicine, its spray is useful in helping sore throat.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Solubility
Soluble in water, alcohol, chloroform, ether, benzene, glycerol, acetone, carbon disulfide and aqueous alkali hydroxides.
Notes
Air and light sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents, strong bases and strong acids.
Soluble in water, alcohol, chloroform, ether, benzene, glycerol, acetone, carbon disulfide and aqueous alkali hydroxides.
Notes
Air and light sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents, strong bases and strong acids.
RUO – Research Use Only
General References:
- Jiang, L.; Jia, T.; Wang, M.; Liao, J.; Cao, P. Pd-Catalyzed Enantioselective Hydroalkoxylation of Alkoxyallenes with Phenol for Construction of Acyclic O, O-Acetals. Org. Lett. 2015, 17 (5), 1070-1073.
- Park, J. H.; Park, C. Y.; Kim, M. J.; Kim, M. U.; Kim, Y. J.; Kim, G. H.; Park, C. P. Continuous-Flow Synthesis of meta-Substituted Phenol Derivatives. Org. Process Res. Dev. 2015, 19 (7), 812-818.