Search Thermo Fisher Scientific
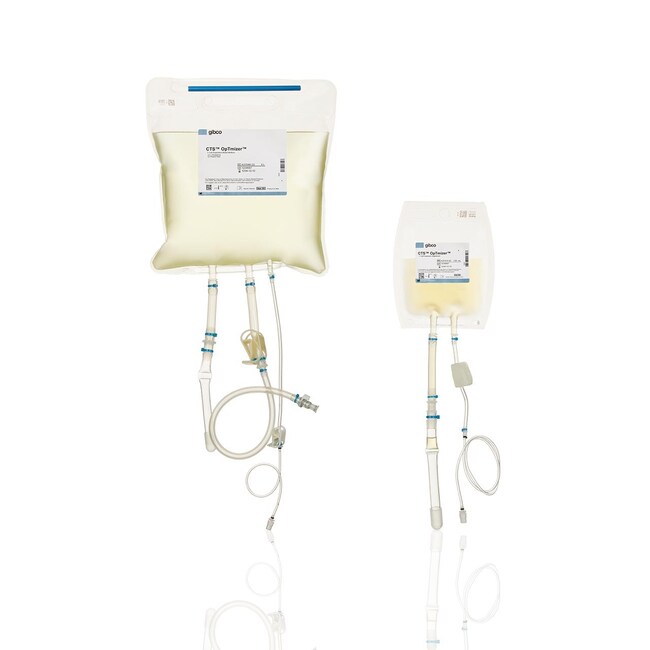
CTS™ OpTmizer™ T-Cell Expansion SFM, no phenol red
| 製品番号(カタログ番号) | 数量 |
|---|---|
| A3705007 | 10 L |
| A3705005 | 5 L |
Gibco CTS OpTmizer T-Cell Expansion SFM supports the growth and expansion of human T lymphocytes. It is a complete serum-free, xeno-free medium consisting of CTS OpTmizer T-Cell Expansion Basal Medium and CTS OpTmizer T-Cell Expansion Supplement, which are mixed together prior to use.
CTS OpTmizer T-Cell Expansion SFM in 5 L and 10 L bag format is compatible with closed-system processing for media preparation. 5 L and 10 L volumes support scaling of cell therapy manufacturing workflows. The updated bag format allows for easier integration with standard cell therapy equipment, including CTS devices (Xenon, Rotea, and DynaCellect).
Features of CTS OpTmizer T-Cell Expansion SFM include:
• Both kit components (medium and supplement) in Aegis5-14 film bags
• Weldable C-Flex and PVC lines
• Supports high-density T-cell culture (>4 x 106 CD3+ T cells/mL)
• Supports T-cell activation using Dynabeads magnetic beads, soluble antibodies, and stimulatory antibody-presenting cell protocols
• Similar phenotype, function (e.g., cytokine secretion profile), and viability to T cells cultured with conventional human AB serum–supplemented medium
• Supports a T-cell phenotype similar to human serum–supplemented medium
• Demonstrates enhanced efficacy and persistence of CART-19 cells when grown in medium supplemented with CTS Immune Cell Serum Replacement (ICSR)
Benefits include:
• Prepare complete medium in a closed system with weldable lines
• Reduces open steps and contamination risks
• Easy integration with CTS automated instrumentation
Demonstrated functional performance
Cells grown in CTS OpTmizer T-Cell Expansion SFM with or without human serum demonstrate good cytokine production profiles in polyclonally activated T cells. CTS OpTmizer T-Cell Expansion SFM maintains the desired the CD62L+ central memory T-cell phenotype.
GMP manufactured
The primary manufacturing site for CTS OpTmizer T-Cell Expansion SFM is FDA-registered and has an ISO13485–certified quality management system. The methods and controls used for the manufacturing are in conformity with current Good Manufacturing Practices for medical devices, 21 CFR Part 820, of the regulation.
Versatility
CTS OpTmizer T-Cell Expansion SFM has been widely adopted in clinical T-cell research applications including the culture of CAR T-cells. It works with multiple culture formats: culture flasks, culture bags, and automated and scalable bioreactors. CTS OpTmizer T-Cell Expansion SFM was designed to support expansion of cultured human T-cells without additional serum supplementation. Increased performance is generally observed when supplemented with CTS Immune Cell Serum Replacement.
• 1 x 260 mL CTS OpTmizer Expansion Supplement in 500 mL bag, store at 2°C to 8°C, protect from light